Chemosphere ( IF 8.1 ) Pub Date : 2020-11-27 , DOI: 10.1016/j.chemosphere.2020.129125 Yejin Li , Linyan Yang , Xueming Chen , Yuefei Han , Guomin Cao
|
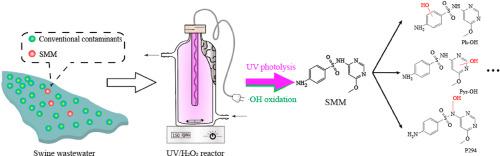
Sulfamonomethoxine (SMM), as one of the most predominant antibiotics in animal wastewater, is pending for effective control to minimize its environmental risks. Transformation kinetics and pathways of SMM by UV/H2O2 in swine wastewater were systematically investigated in this study. Direct UV photolysis (as a dominant role) and ∙OH oxidation contributed to SMM degradation in UV/H2O2 system. The less effective reaction rate of SMM in real wastewater than synthetic wastewater (0.1-0.17 vs. ∼0.2-1.5 min-1, despite higher H2O2 dosage and extended reaction time) resulted mainly from the abundant presence of conventional contaminants (indicated by COD, a notable competitor of SMM) in real wastewater. SMM degradation benefited from higher H2O2 dosage and neutral and weak alkaline conditions. However, the effect of initial SMM concentration on SMM degradation in synthetic and real wastewater showed opposite trends, owning to the different probability of SMM molecules to interact with UV and H2O2 in different matrices. Carbonate had an inhibitory effect on SMM degradation by scavenging ∙OH and pH-variation induced effect, while nitrate promoted SMM degradation by generating more ∙OH. The removal efficiency of SMM in real wastewater reached 91% under the reaction conditions of H2O2 of 10 mM, reaction time of 60 min, and pH 6.7-6.9. SMM degradation pathway was proposed as hydroxylation of benzene and pyrimidine rings, and secondary amine, and the subsequent cleavage of S-N bond.
中文翻译:

UV / H 2 O 2在猪废水中磺胺甲硫辛的转化动力学及途径
磺胺二甲氧嘧啶(SMM)作为动物废水中最主要的抗生素之一,正在等待有效控制以最小化其环境风险。本研究系统地研究了UV / H 2 O 2在猪废水中SMM的转化动力学和途径。直接的紫外线光解(起主要作用)和∙OH氧化导致SMM在UV / H 2 O 2系统中降解。尽管H 2 O 2更高,但实际废水中SMM的有效反应速率却低于合成废水(0.1-0.17 vs.约0.2-1.5 min -1)剂量和延长的反应时间)主要是由于实际废水中大量存在常规污染物(以SMM的著名竞争对手COD表示)。SMM降解得益于更高的H 2 O 2剂量以及中性和弱碱性条件。然而,由于SMM分子与UV和H 2 O 2相互作用的可能性不同,初始SMM浓度对合成废水和实际废水中SMM降解的影响呈现相反的趋势。在不同的矩阵中 碳酸盐通过清除∙OH和pH变化诱导的作用对SMM降解具有抑制作用,而硝酸盐则通过生成更多的∙OH来促进SMM降解。在H 2 O 2为10 mM,反应时间为60 min,pH为6.7-6.9的条件下,实际废水中SMM的去除效率达到91%。SMM降解途径被提议为苯和嘧啶环以及仲胺的羟基化,以及随后的SN键裂解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号