Biomaterials ( IF 14.0 ) Pub Date : 2020-11-27 , DOI: 10.1016/j.biomaterials.2020.120581 Yue Huang 1 , Yuan Liu 2 , Shrey Shah 3 , Dongyeop Kim 4 , Aurea Simon-Soro 2 , Tatsuro Ito 5 , Maryam Hajfathalian 6 , Yong Li 7 , Jessica C Hsu 3 , Lenitza M Nieves 6 , Faizan Alawi 8 , Pratap C Naha 6 , David P Cormode 9 , Hyun Koo 10
|
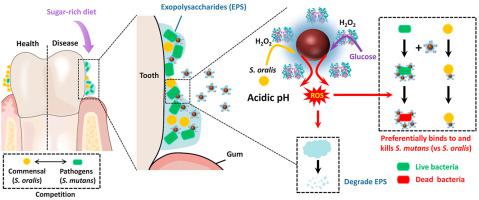
Human dental caries is an intractable biofilm-associated disease caused by microbial interactions and dietary sugars on the host's teeth. Commensal bacteria help control opportunistic pathogens via bioactive products such as hydrogen peroxide (H2O2). However, high-sugar consumption disrupts homeostasis and promotes pathogen accumulation in acidic biofilms that cause tooth-decay. Here, we exploit the pathological (sugar-rich/acidic) conditions using a nanohybrid system to increase intrinsic H2O2 production and trigger pH-dependent reactive oxygen species (ROS) generation for efficient biofilm virulence targeting. The nanohybrid contains glucose-oxidase that catalyzes glucose present in biofilms to increase intrinsic H2O2, which is converted by iron oxide nanoparticles with peroxidase-like activity into ROS in acidic pH. Notably, it selectively kills Streptococcus mutans (pathogen) without affecting Streptococcus oralis (commensal) via preferential pathogen-binding and in situ ROS generation. Furthermore, nanohybrid treatments potently reduced dental caries in a rodent model. Compared to chlorhexidine (positive-control), which disrupted oral microbiota diversity, the nanohybrid had significant higher efficacy without affecting soft-tissues and the oral-gastrointestinal microbiomes, while modulating dental health-associated microbial activity in vivo. The data reveal therapeutic precision of a bi-functional hybrid nanozyme against a biofilm-related disease in a controlled-manner activated by pathological conditions.
中文翻译:

通过生物膜微环境激活的双功能纳米酶精确靶向细菌病原体
人类龋齿是一种顽固的生物膜相关疾病,由宿主牙齿上的微生物相互作用和膳食糖分引起。共生细菌通过过氧化氢 (H 2 O 2 )等生物活性产品帮助控制机会性病原体。然而,高糖消耗会破坏体内平衡并促进病原体在导致蛀牙的酸性生物膜中的积累。在这里,我们使用纳米混合系统利用病理(富含糖/酸性)条件来增加内在 H 2 O 2产生并触发 pH 依赖性活性氧 (ROS) 生成,以实现有效的生物膜毒力靶向。纳米杂化物含有葡萄糖氧化酶,可催化生物膜中存在的葡萄糖增加内在 H 2 O 2,在酸性 pH 值下,H 2 O 2被具有过氧化物酶样活性的氧化铁纳米颗粒转化为 ROS。值得注意的是,它通过优先病原体结合和原位选择性杀死变形链球菌(病原体)而不影响口腔链球菌(共生)ROS 生成。此外,纳米混合治疗有效地减少了啮齿动物模型中的龋齿。与破坏口腔微生物群多样性的氯己定(阳性对照)相比,纳米混合物具有显着更高的功效,同时不影响软组织和口腔胃肠道微生物群,同时调节体内与牙齿健康相关的微生物活性。数据揭示了双功能杂化纳米酶以由病理条件激活的受控方式对抗生物膜相关疾病的治疗精度。



























 京公网安备 11010802027423号
京公网安备 11010802027423号