当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral cyclometalated iridium complexes for asymmetric reduction reactions
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-11-23 , DOI: 10.1039/d0ob02049d Jennifer Smith 1, 2, 3, 4 , Aysecik Kacmaz 1, 2, 3, 4 , Chao Wang 1, 2, 3, 4 , Barbara Villa-Marcos 1, 2, 3, 4 , Jianliang Xiao 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-11-23 , DOI: 10.1039/d0ob02049d Jennifer Smith 1, 2, 3, 4 , Aysecik Kacmaz 1, 2, 3, 4 , Chao Wang 1, 2, 3, 4 , Barbara Villa-Marcos 1, 2, 3, 4 , Jianliang Xiao 1, 2, 3, 4
Affiliation
|
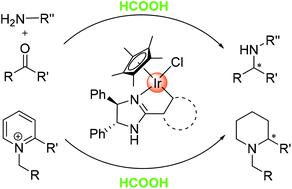
A series of chiral cyclometalated iridium complexes have been synthesised by cyclometalating chiral 2-aryl-oxazoline and imidazoline ligands with [Cp*IrCl2]2. These iridacycles were studied for asymmetric transfer hydrogenation reactions with formic acid as the hydrogen source and were found to display various activities and enantioselectivities, with the most effective ones affording up to 63% ee in the asymmetric reductive amination of ketones and 77% ee in the reduction of pyridinium ions.
中文翻译:

手性环金属铱配合物用于不对称还原反应
通过将手性2-芳基-恶唑啉和咪唑啉配体与[Cp * IrCl 2 ] 2进行环化,合成了一系列手性环金属化铱配合物。对这些iridacycles进行了以甲酸为氢源的不对称转移氢化反应的研究,发现它们具有各种活性和对映选择性,最有效的酮类化合物在酮的不对称还原胺化反应中可提供高达63%ee的活性,而在酮中则可提供77%ee的活性。还原吡啶鎓离子。
更新日期:2020-11-27
中文翻译:

手性环金属铱配合物用于不对称还原反应
通过将手性2-芳基-恶唑啉和咪唑啉配体与[Cp * IrCl 2 ] 2进行环化,合成了一系列手性环金属化铱配合物。对这些iridacycles进行了以甲酸为氢源的不对称转移氢化反应的研究,发现它们具有各种活性和对映选择性,最有效的酮类化合物在酮的不对称还原胺化反应中可提供高达63%ee的活性,而在酮中则可提供77%ee的活性。还原吡啶鎓离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号