当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Dr Jekyll and Mr Hyde of lithium sulfur batteries
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2020-11-26 , DOI: 10.1039/d0ee02797a Patrick Bonnick 1, 2, 3 , John Muldoon 1, 2, 3
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2020-11-26 , DOI: 10.1039/d0ee02797a Patrick Bonnick 1, 2, 3 , John Muldoon 1, 2, 3
Affiliation
|
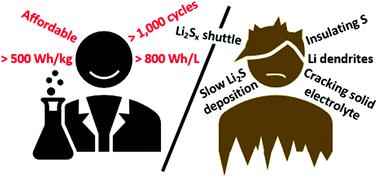
Although the concept of a lithium–sulfur (Li–S) battery promises an energy density surpassing that of conventional Li-ion cells, prototype cells have lagged far behind. As research on Li–S has progressed, four limiting challenges for the sulfur electrode have now emerged that must be addressed to facilitate their realization, including slow lithium polysulfide deposition kinetics at low electrolyte/sulfur ratios, the lithium polysulfide shuttle, the low electrical conductivity of sulfur active material, and the cracking of solid electrolytes due to the repeated expansion and contraction of sulfur active material. Notably, the challenges of both slow deposition kinetics and the lithium polysulfide shuttle only arise when using sulfur active material with the most common electrolyte solvents used in Li–S cells: liquid ethereal mixtures, such as DOL:DME. Consequently, a reckoning of ether-based electrolytes is at hand, which has shifted research focus toward solid electrolytes and alternative sulfur-based active materials; however, these alternative strategies have their own challenges. Meanwhile, dendrite growth and insufficient coulombic efficiency still plague lithium metal electrodes in both liquid and solid electrolytes at relevant current densities. Many approaches to mitigate dendrite growth have been attempted, including artificial SEIs, nucleation aids, high surface area current collectors and solid electrolytes. Despite successes, further work is needed to attain a sufficient level of performance for lithium metal to be used in commercial Li–S cells. Herein we discuss the strengths and weaknesses of many proposed solutions to these challenges with an eye toward the ever-present goal of competing with conventional Li-ion energy densities.
中文翻译:

锂硫电池的Jekyll博士和Hyde先生
尽管锂硫(Li–S)电池的概念有望使能量密度超过传统的锂离子电池,但原型电池却远远落后。随着对Li–S的研究的发展,硫电极的四个局限性挑战已经出现,必须加以解决以促进其实现,包括在低电解质/硫比下缓慢的多硫化锂沉积动力学,多硫化锂梭,低电导率硫活性材料的分解,以及由于硫活性材料的反复膨胀和收缩而导致的固体电解质的裂解。值得注意的是,只有在将硫磺活性材料与Li-S电池中使用的最常见的电解质溶剂(如DOL等液体醚混合物)一起使用时,沉积动力学和多硫化锂往复运动的挑战才出现。DME。因此,对基于醚的电解质的估算即将到来,这已将研究重点转向了固体电解质和替代性的基于硫的活性材料。但是,这些替代策略有其自身的挑战。同时,在相关电流密度下,液体和固体电解质中的枝晶生长和不足的库仑效率仍然困扰着锂金属电极。已经尝试了许多减轻枝晶生长的方法,包括人造SEI,成核助剂,高表面积集电器和固体电解质。尽管取得了成功,但仍需要进一步的工作以使锂金属在商用Li-S电池中使用时达到足够的性能水平。
更新日期:2020-11-27
中文翻译:

锂硫电池的Jekyll博士和Hyde先生
尽管锂硫(Li–S)电池的概念有望使能量密度超过传统的锂离子电池,但原型电池却远远落后。随着对Li–S的研究的发展,硫电极的四个局限性挑战已经出现,必须加以解决以促进其实现,包括在低电解质/硫比下缓慢的多硫化锂沉积动力学,多硫化锂梭,低电导率硫活性材料的分解,以及由于硫活性材料的反复膨胀和收缩而导致的固体电解质的裂解。值得注意的是,只有在将硫磺活性材料与Li-S电池中使用的最常见的电解质溶剂(如DOL等液体醚混合物)一起使用时,沉积动力学和多硫化锂往复运动的挑战才出现。DME。因此,对基于醚的电解质的估算即将到来,这已将研究重点转向了固体电解质和替代性的基于硫的活性材料。但是,这些替代策略有其自身的挑战。同时,在相关电流密度下,液体和固体电解质中的枝晶生长和不足的库仑效率仍然困扰着锂金属电极。已经尝试了许多减轻枝晶生长的方法,包括人造SEI,成核助剂,高表面积集电器和固体电解质。尽管取得了成功,但仍需要进一步的工作以使锂金属在商用Li-S电池中使用时达到足够的性能水平。



























 京公网安备 11010802027423号
京公网安备 11010802027423号