当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Proton-Coupled Electron Transfer for Photoinduced Generation of Two-Electron Reduced Species of Quinone
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-26 , DOI: 10.1021/acs.jpcb.0c07809 Ananta Dey 1, 2 , Nandan Ghorai 3 , Amitava Das 2, 4 , Hirendra N. Ghosh 3, 5
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-26 , DOI: 10.1021/acs.jpcb.0c07809 Ananta Dey 1, 2 , Nandan Ghorai 3 , Amitava Das 2, 4 , Hirendra N. Ghosh 3, 5
Affiliation
|
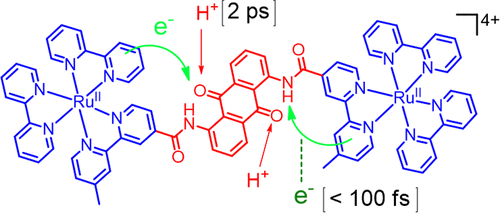
Purpose-built molecules that follow the fundamental process of photosynthesis have significance in developing better insight into the natural photosynthesis process. Quinones have a significant role as electron acceptors in natural photosynthesis, and their reduction is assisted through H-bond donation or protonation. The major challenge in such studies is to couple the multielectron and proton-transfer process and to achieve a reasonably stable charge-separated state for the elucidation of the mechanistic pathway. We have tried to address this issue through the design of a donor–acceptor–donor molecular triad (2RuAQ) derived from two equivalent [Ru(bpy)3]2+ derivatives and a bridging anthraquinone moiety (AQ). Photoinduced proton-coupled electron transfer (PCET) for this molecular triad was systematically investigated in the absence and presence of hexafluoroisopropanol and p-toluenesulfonic acid (PTSA) using time-resolved absorption spectroscopy in the ultrafast time domain. Results reveal the generation of a relatively long-lived charge-separated state in this multi-electron transfer reaction, and we could confirm the generation of AQ2– and RuIII as the transient intermediates. We could rationalize the mechanistic pathway and the dynamics associated with photoinduced processes and the role of H-bonding in stabilizing charge-separated states. Transient absorption spectroscopic studies reveal that the rates of intramolecular electron transfer and the mechanistic pathways associated with the PCET process are significantly different in different solvent compositions having different polarities. In acetonitrile, a concerted PCET mechanism prevails, whereas the stepwise PCET reaction process is observed in the presence of PTSA. The results of the present study represent a unique model for the mechanistic diversity of PCET reactions.
中文翻译:

质子耦合电子转移用于光诱导生成两电子还原的醌
遵循光合作用的基本过程的专门构建的分子对于深入了解自然光合作用过程具有重要意义。醌在自然光合作用中起着重要的电子受体的作用,而氢键的键合或质子化有助于其还原。在此类研究中的主要挑战是将多电子和质子转移过程耦合在一起,并为阐明机理途径实现合理稳定的电荷分离状态。我们尝试通过设计来自两个等效[Ru(bpy)3 ] 2+的供体-受体-供体分子三元组(2RuAQ)来解决此问题。衍生物和桥接蒽醌部分(AQ)。在六氟异丙醇和对甲苯磺酸(PTSA)不存在和存在的情况下,使用超快时域中的时间分辨吸收光谱系统地研究了该分子三元组的光诱导质子偶联电子转移(PCET)。结果表明,在这种多电子转移反应中,生成了一个寿命较长的电荷分离态,我们可以确定生成了AQ 2–和Ru III。作为过渡中间体。我们可以合理化机械途径和与光诱导过程相关的动力学,以及氢键在稳定电荷分离态中的作用。瞬态吸收光谱研究表明,在具有不同极性的不同溶剂组合物中,分子内电子转移的速率和与PCET过程相关的机理途径显着不同。在乙腈中,普遍存在协同作用的PCET机制,而在PTSA存在下观察到逐步PCET反应过程。本研究的结果代表了PCET反应机理多样性的独特模型。
更新日期:2020-12-10
中文翻译:

质子耦合电子转移用于光诱导生成两电子还原的醌
遵循光合作用的基本过程的专门构建的分子对于深入了解自然光合作用过程具有重要意义。醌在自然光合作用中起着重要的电子受体的作用,而氢键的键合或质子化有助于其还原。在此类研究中的主要挑战是将多电子和质子转移过程耦合在一起,并为阐明机理途径实现合理稳定的电荷分离状态。我们尝试通过设计来自两个等效[Ru(bpy)3 ] 2+的供体-受体-供体分子三元组(2RuAQ)来解决此问题。衍生物和桥接蒽醌部分(AQ)。在六氟异丙醇和对甲苯磺酸(PTSA)不存在和存在的情况下,使用超快时域中的时间分辨吸收光谱系统地研究了该分子三元组的光诱导质子偶联电子转移(PCET)。结果表明,在这种多电子转移反应中,生成了一个寿命较长的电荷分离态,我们可以确定生成了AQ 2–和Ru III。作为过渡中间体。我们可以合理化机械途径和与光诱导过程相关的动力学,以及氢键在稳定电荷分离态中的作用。瞬态吸收光谱研究表明,在具有不同极性的不同溶剂组合物中,分子内电子转移的速率和与PCET过程相关的机理途径显着不同。在乙腈中,普遍存在协同作用的PCET机制,而在PTSA存在下观察到逐步PCET反应过程。本研究的结果代表了PCET反应机理多样性的独特模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号