当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Delving into Autocatalytic Liquid-State Thermal Decomposition of Novel Energetic 1,3,5-Triazines with Azido, Trinitroethyl, and Nitramino Groups
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-26 , DOI: 10.1021/acs.jpcb.0c08259 Nikita V. Muravyev 1 , Viktor V. Zakharov 2 , Boris L. Korsounskii 1, 2 , Konstantin A. Monogarov 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-26 , DOI: 10.1021/acs.jpcb.0c08259 Nikita V. Muravyev 1 , Viktor V. Zakharov 2 , Boris L. Korsounskii 1, 2 , Konstantin A. Monogarov 1
Affiliation
|
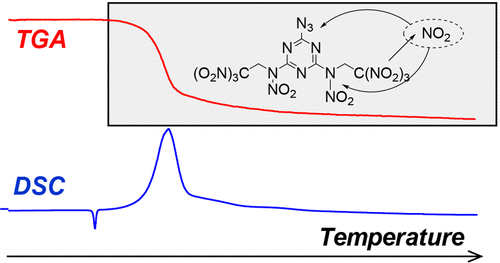
Thermal decomposition of 1,3,5-triazines with azido, trinitroethyl, and nitramino groups, the three important energetic functionalities, has been studied with a range of thermal analysis tools. The involved compounds melt under heating with the following mass loss and heat and gas release in the course of thermal decomposition. Model-fitting kinetic analysis resulted in formal reaction schemes with two general stages. In case of the least energetic 6-azido-2,4-bis(2,2,2-trinitroethylamino)-1,3,5-triazine, the first reaction is a first-order reaction followed by a third-order reaction. Alternatively, for 6-azido-2,4-bis(2,2,2-trinitroethylnitramino)-1,3,5-triazine and 2,4,6-tris(2,2,2-trinitroethylnitramino)-1,3,5-triazine, the first step comprises the autocatalytic reaction. The activation energy for the first decomposition step drops from 141 to 122 kJ mol–1 due to the inductive influence of a β-nitramino group. The second general reaction for all species obeys the third-order reaction model with activation energies in the range 112–126 kJ mol–1. On the basis of the analysis of the kinetic data and temporal behavior of the evolved gases, a similar primary decomposition channel, the homolytic cleavage of a C–NO2 bond, has been proposed for all investigated substances.
中文翻译:

研究具有叠氮基,三硝基乙基和硝基氨基的新型高能1,3,5-三嗪的自催化液相热分解
已使用一系列热分析工具研究了具有叠氮基,三硝基乙基和硝氨基的1,3,5-三嗪的热分解,这是三个重要的能量功能。所涉及的化合物在加热下熔融,随后损失质量,并且在热分解过程中释放出热量和气体。模型拟合动力学分析得出了具有两个大致阶段的正式反应方案。在能量最低的6-叠氮基-2,4-双(2,2,2-三硝基乙基氨基)-1,3,5-三嗪的情况下,第一反应是第一级反应,然后是第三级反应。或者,对于6-叠氮基-2,4-双(2,2,2-三硝基乙基硝酸氨基)-1,3,5-三嗪和2,4,6-三(2,2,2-三硝基乙基硝酸氨基)-1,3 ,5-三嗪,第一步包括自催化反应。–1由于β-硝胺基的感应作用。所有物种的第二个一般反应服从三阶反应模型,其活化能在112–126 kJ mol –1范围内。在分析所释放出的气体的动力学数据和时间行为的基础上,对所有被研究的物质都提出了类似的主要分解通道,即C–NO 2键的均质裂解。
更新日期:2020-12-10
中文翻译:

研究具有叠氮基,三硝基乙基和硝基氨基的新型高能1,3,5-三嗪的自催化液相热分解
已使用一系列热分析工具研究了具有叠氮基,三硝基乙基和硝氨基的1,3,5-三嗪的热分解,这是三个重要的能量功能。所涉及的化合物在加热下熔融,随后损失质量,并且在热分解过程中释放出热量和气体。模型拟合动力学分析得出了具有两个大致阶段的正式反应方案。在能量最低的6-叠氮基-2,4-双(2,2,2-三硝基乙基氨基)-1,3,5-三嗪的情况下,第一反应是第一级反应,然后是第三级反应。或者,对于6-叠氮基-2,4-双(2,2,2-三硝基乙基硝酸氨基)-1,3,5-三嗪和2,4,6-三(2,2,2-三硝基乙基硝酸氨基)-1,3 ,5-三嗪,第一步包括自催化反应。–1由于β-硝胺基的感应作用。所有物种的第二个一般反应服从三阶反应模型,其活化能在112–126 kJ mol –1范围内。在分析所释放出的气体的动力学数据和时间行为的基础上,对所有被研究的物质都提出了类似的主要分解通道,即C–NO 2键的均质裂解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号