当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protein Side-Chain–DNA Contacts Probed by Fast Magic-Angle Spinning NMR
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jpcb.0c08150 Denis Lacabanne 1 , Julien Boudet 2 , Alexander A. Malär 1 , Pengzhi Wu 2, 3 , Riccardo Cadalbert 1 , Loic Salmon 2 , Frédéric H.-T. Allain 2, 3 , Beat H. Meier 1 , Thomas Wiegand 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jpcb.0c08150 Denis Lacabanne 1 , Julien Boudet 2 , Alexander A. Malär 1 , Pengzhi Wu 2, 3 , Riccardo Cadalbert 1 , Loic Salmon 2 , Frédéric H.-T. Allain 2, 3 , Beat H. Meier 1 , Thomas Wiegand 1
Affiliation
|
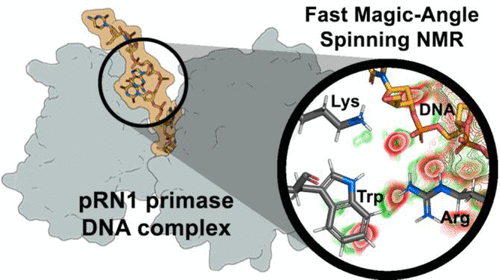
Protein–nucleic acid interactions are essential in a variety of biological events ranging from the replication of genomic DNA to the synthesis of proteins. Noncovalent interactions guide such molecular recognition events, and protons are often at the center of them, particularly due to their capability of forming hydrogen bonds to the nucleic acid phosphate groups. Fast magic-angle spinning experiments (100 kHz) reduce the proton NMR line width in solid-state NMR of fully protonated protein–DNA complexes to such an extent that resolved proton signals from side-chains coordinating the DNA can be detected. We describe a set of NMR experiments focusing on the detection of protein side-chains from lysine, arginine, and aromatic amino acids and discuss the conclusions that can be obtained on their role in DNA coordination. We studied the 39 kDa enzyme of the archaeal pRN1 primase complexed with DNA and characterize protein–DNA contacts in the presence and absence of bound ATP molecules.
中文翻译:

快速魔术角旋转NMR探测蛋白质的侧链– DNA接触
在从基因组DNA复制到蛋白质合成的各种生物学事件中,蛋白质-核酸相互作用都是必不可少的。非共价相互作用指导这种分子识别事件,并且质子通常位于它们的中心,特别是由于其形成与核酸磷酸基团形成氢键的能力。快速魔角旋转实验(100 kHz)将完全质子化的蛋白质-DNA复合物的固态NMR中的质子NMR线宽度减小到可以检测到来自与DNA配位的侧链的已分解质子信号的程度。我们描述了一组NMR实验,重点是从赖氨酸,精氨酸和芳香族氨基酸中检测蛋白质侧链,并讨论了在DNA配位中可以得出的结论。
更新日期:2020-12-10
中文翻译:

快速魔术角旋转NMR探测蛋白质的侧链– DNA接触
在从基因组DNA复制到蛋白质合成的各种生物学事件中,蛋白质-核酸相互作用都是必不可少的。非共价相互作用指导这种分子识别事件,并且质子通常位于它们的中心,特别是由于其形成与核酸磷酸基团形成氢键的能力。快速魔角旋转实验(100 kHz)将完全质子化的蛋白质-DNA复合物的固态NMR中的质子NMR线宽度减小到可以检测到来自与DNA配位的侧链的已分解质子信号的程度。我们描述了一组NMR实验,重点是从赖氨酸,精氨酸和芳香族氨基酸中检测蛋白质侧链,并讨论了在DNA配位中可以得出的结论。



























 京公网安备 11010802027423号
京公网安备 11010802027423号