当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determinant Factor for Thermodynamic Stability of Sulfuric Acid–Amine Complexes
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jpca.0c07908 Jia Han 1 , Lei Wang 1 , Hanhui Zhang 1 , Quyan Su 1 , Xiaoguo Zhou 1 , Shilin Liu 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jpca.0c07908 Jia Han 1 , Lei Wang 1 , Hanhui Zhang 1 , Quyan Su 1 , Xiaoguo Zhou 1 , Shilin Liu 1
Affiliation
|
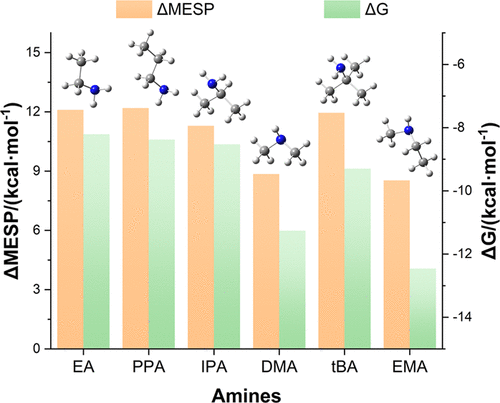
Atmospheric amines are thought to play significant roles in the nucleation of sulfuric acid-mediated aerosol particles. Their enhancing effects on the stabilization of the related complexes have formerly been correlated with the amine base strength, but there are a few exceptions reported. In this work, the influence of seven alkylamines on the thermodynamic stability of sulfuric acid–amine complexes has been theoretically investigated, e.g., ethylamine, propylamine, isopropylamine, tert-butylamine, dimethylamine, ethylmethylamine, and trimethylamine. For all primary and secondary amine-mediated complexes, a dual hydrogen bond configuration is generally suggested in the most stable isomer. The stabilization of this special structure predicted by the electrostatic potential distribution on the molecular surface of amines exactly agrees with the base strength sequence, providing crucial evidence for the previous deduction of correlation between the base strength and the enhancing effect. Meanwhile, the considerable van der Waals interactions are found between the free hydroxyl of sulfuric acid and the β-methyl group of amine, resulting in the extra stability for sulfuric acid–dimethylamine and sulfuric acid–ethylmethylamine complexes. Therefore, the electrostatic potential distribution of amines is the essential determinant factor for the thermodynamic stability of the relevant complexes. Our conclusions provide new insight into a way to evaluate the enhancing abilities of amines in aerosol particle nucleation.
中文翻译:

硫酸-胺配合物热力学稳定性的决定因素
人们认为,大气胺在硫酸介导的气溶胶颗粒成核中起重要作用。它们对相关配合物的稳定作用的增强作用以前与胺碱强度有关,但有一些例外报道。在这项工作中,对硫酸-胺络合物的热力学稳定性7个烷基胺的影响已经从理论上研究,例如,乙胺,丙胺,异丙胺,叔-丁胺,二甲胺,乙基甲胺和三甲胺。对于所有伯胺和仲胺介导的络合物,通常建议在最稳定的异构体中使用双氢键构型。由胺分子表面上的静电势分布预测的这种特殊结构的稳定性与碱强度序列完全吻合,为先前推断出碱强度和增强作用之间的相关性提供了重要证据。同时,在硫酸的游离羟基和胺的β-甲基之间发现了显着的范德华相互作用,从而为硫酸-二甲胺和硫酸-乙基甲胺络合物提供了额外的稳定性。因此,胺的静电势分布是相关配合物热力学稳定性的重要决定因素。我们的结论为评估胺在气溶胶颗粒成核中的增强能力提供了新的见解。
更新日期:2020-12-10
中文翻译:

硫酸-胺配合物热力学稳定性的决定因素
人们认为,大气胺在硫酸介导的气溶胶颗粒成核中起重要作用。它们对相关配合物的稳定作用的增强作用以前与胺碱强度有关,但有一些例外报道。在这项工作中,对硫酸-胺络合物的热力学稳定性7个烷基胺的影响已经从理论上研究,例如,乙胺,丙胺,异丙胺,叔-丁胺,二甲胺,乙基甲胺和三甲胺。对于所有伯胺和仲胺介导的络合物,通常建议在最稳定的异构体中使用双氢键构型。由胺分子表面上的静电势分布预测的这种特殊结构的稳定性与碱强度序列完全吻合,为先前推断出碱强度和增强作用之间的相关性提供了重要证据。同时,在硫酸的游离羟基和胺的β-甲基之间发现了显着的范德华相互作用,从而为硫酸-二甲胺和硫酸-乙基甲胺络合物提供了额外的稳定性。因此,胺的静电势分布是相关配合物热力学稳定性的重要决定因素。我们的结论为评估胺在气溶胶颗粒成核中的增强能力提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号