当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Expanding the Scope of Palladium-Catalyzed B–N Cross-Coupling Chemistry in Carboranes
Organometallics ( IF 2.5 ) Pub Date : 2020-11-26 , DOI: 10.1021/acs.organomet.0c00576 Xin Mu 1 , Morgan Hopp 1 , Rafal M Dziedzic 1 , Mary A Waddington 1 , Arnold L Rheingold 2 , Ellen M Sletten 1 , Jonathan C Axtell 1 , Alexander M Spokoyny 1
Organometallics ( IF 2.5 ) Pub Date : 2020-11-26 , DOI: 10.1021/acs.organomet.0c00576 Xin Mu 1 , Morgan Hopp 1 , Rafal M Dziedzic 1 , Mary A Waddington 1 , Arnold L Rheingold 2 , Ellen M Sletten 1 , Jonathan C Axtell 1 , Alexander M Spokoyny 1
Affiliation
|
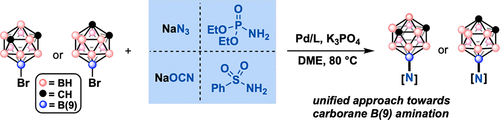
Over the past several years, a number of strategies for the functionalization of dicarba-closo-dodecaboranes (carboranes) have emerged. Despite these developments, B–N bond formation on the carborane scaffold remains a challenge due to the propensity of strong nucleophiles to partially deboronate the parent closo-carborane cluster into the corresponding nido form. Here we show that azide, sulfonamide, cyanate, and phosphoramidate nucleophiles can be straightforwardly cross-coupled onto the B(9) vertices of the o- and m-carborane core from readily accessible precursors without significant deboronation byproducts, laying the groundwork for further study into the utility and properties of these new B-aminated carborane species. We further showcase the select reactivity of the installed functional groups, highlighting some unique features stemming from the combination of the electron-donating B(9) position and the large steric profile of the B-connected carborane substituent.
中文翻译:

扩大碳硼烷中钯催化 B-N 交叉偶联化学的范围
在过去的几年中,许多用于dicarba-的功能化战略闭合碳-dodecaboranes(碳硼烷)已经出现。尽管有这些进展,对碳硼烷骨架B-N键的形成仍然是一个挑战,由于强的亲核试剂的倾向部分deboronate父闭合碳-carborane簇成相应的巢式形式。在这里,我们表明叠氮化物、磺酰胺、氰酸盐和氨基磷酸酯亲核试剂可以直接交叉耦合到o - 和m的 B(9) 顶点-碳硼烷核心来自易于获得的前体,没有显着的脱硼副产物,为进一步研究这些新的 B-胺化碳硼烷物种的效用和性质奠定了基础。我们进一步展示了安装的官能团的选择性反应性,突出了一些独特的特征,这些特征源于供电子 B(9) 位置和 B 连接碳硼烷取代基的大空间分布的组合。
更新日期:2020-12-14
中文翻译:

扩大碳硼烷中钯催化 B-N 交叉偶联化学的范围
在过去的几年中,许多用于dicarba-的功能化战略闭合碳-dodecaboranes(碳硼烷)已经出现。尽管有这些进展,对碳硼烷骨架B-N键的形成仍然是一个挑战,由于强的亲核试剂的倾向部分deboronate父闭合碳-carborane簇成相应的巢式形式。在这里,我们表明叠氮化物、磺酰胺、氰酸盐和氨基磷酸酯亲核试剂可以直接交叉耦合到o - 和m的 B(9) 顶点-碳硼烷核心来自易于获得的前体,没有显着的脱硼副产物,为进一步研究这些新的 B-胺化碳硼烷物种的效用和性质奠定了基础。我们进一步展示了安装的官能团的选择性反应性,突出了一些独特的特征,这些特征源于供电子 B(9) 位置和 B 连接碳硼烷取代基的大空间分布的组合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号