当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P2Y12 antagonism results in altered interactions between platelets and regulatory T cells during sepsis
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-11-26 , DOI: 10.1002/jlb.3a0220-097r Samara Albayati 1 , Harika Vemulapalli 1 , Alexander Y Tsygankov 1, 2 , Elisabetta Liverani 1
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2020-11-26 , DOI: 10.1002/jlb.3a0220-097r Samara Albayati 1 , Harika Vemulapalli 1 , Alexander Y Tsygankov 1, 2 , Elisabetta Liverani 1
Affiliation
|
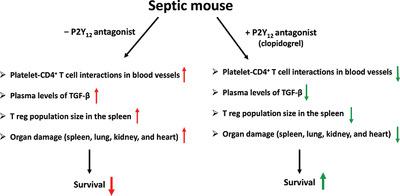
Sepsis is a complex clinical condition resulting from a serious bloodstream infection. With mortality rates as high as 50%, improved treatments are needed. Regulatory T cells (Tregs), a subset of T lymphocytes, promote the resolution of inflammation. Septic patients have elevated levels of circulating Tregs. Platelets influence the proliferation and activation of Tregs in vitro. However, modulating platelet-Tregs interaction during sepsis may restraing Treg proliferation, leading to the restoration of immunologic homeostasis. P2Y12 is a purinergic receptor present on platelets and T lymphocytes. Blocking P2Y12 improves the outcome of sepsis. We investigated whether blocking P2Y12 alters platelet–Treg interaction in vivo. We used the murine model of sepsis, cecal ligation, and puncture (CLP) and we blocked P2Y12 using the P2Y12 antagonist, clopidogrel. Twenty-four hours after surgery, we measured Treg population sizes in the spleens of the Sham, CLP, and CLP + clopidogrel groups. We investigated the effect of blocking P2Y12 in vitro using cocultures of human platelets and T cells with or without anti-CD3/CD28. P2Y12 was blocked using AR-C69931MX. Treg population sizes were reduced in the septic mice treated with clopidogrel compared with untreated septic mice. Aggregation of platelets and CD4+ T cells was reduced in treated CLP mice compared with untreated CLP mice. P2Y12 antagonism changes how platelets influence T cells in vitro, depending on T-cell activation. In conclusion, blockade of the P2Y12 signaling pathway restrains Treg proliferation in vivo and in vitro. Targeting platelets to control Treg proliferation and activity may be a promising strategy for treating sepsis.
中文翻译:

P2Y12 拮抗作用导致败血症期间血小板和调节性 T 细胞之间的相互作用发生改变
脓毒症是一种由严重血流感染引起的复杂临床病症。由于死亡率高达 50%,因此需要改进治疗方法。调节性 T 细胞 (Tregs) 是 T 淋巴细胞的一个子集,可促进炎症的消退。脓毒症患者的循环 Treg 水平升高。血小板影响体外 Tregs 的增殖和活化。然而,在脓毒症期间调节血小板-Treg 相互作用可能会抑制 Treg 增殖,导致免疫稳态的恢复。P2Y 12是一种存在于血小板和 T 淋巴细胞上的嘌呤能受体。阻断 P2Y 12可改善败血症的结果。我们调查了是否阻断 P2Y 12改变体内血小板-Treg相互作用。我们使用败血症、盲肠结扎和穿刺 (CLP) 的鼠模型,并使用 P2Y 12拮抗剂氯吡格雷阻断 P2Y 12。手术后 24 小时,我们测量了 Sham、CLP 和 CLP + 氯吡格雷组脾脏中 Treg 群体的大小。我们使用人血小板和 T 细胞的共培养物在有或没有抗 CD3/CD28 的情况下研究了体外阻断 P2Y 12的效果。使用 AR-C69931MX 阻止了P2Y 12。与未治疗的脓毒症小鼠相比,用氯吡格雷治疗的脓毒症小鼠的 Treg 种群大小减少。与未经处理的 CLP 小鼠相比,经处理的 CLP 小鼠的血小板和 CD4 + T 细胞的聚集减少。P2Y12拮抗作用会改变血小板在体外影响 T 细胞的方式,这取决于 T 细胞的激活。总之,P2Y 12信号通路的阻断抑制了体内和体外的 Treg 增殖。靶向血小板以控制 Treg 增殖和活性可能是治疗脓毒症的一种有前景的策略。
更新日期:2020-11-26
中文翻译:

P2Y12 拮抗作用导致败血症期间血小板和调节性 T 细胞之间的相互作用发生改变
脓毒症是一种由严重血流感染引起的复杂临床病症。由于死亡率高达 50%,因此需要改进治疗方法。调节性 T 细胞 (Tregs) 是 T 淋巴细胞的一个子集,可促进炎症的消退。脓毒症患者的循环 Treg 水平升高。血小板影响体外 Tregs 的增殖和活化。然而,在脓毒症期间调节血小板-Treg 相互作用可能会抑制 Treg 增殖,导致免疫稳态的恢复。P2Y 12是一种存在于血小板和 T 淋巴细胞上的嘌呤能受体。阻断 P2Y 12可改善败血症的结果。我们调查了是否阻断 P2Y 12改变体内血小板-Treg相互作用。我们使用败血症、盲肠结扎和穿刺 (CLP) 的鼠模型,并使用 P2Y 12拮抗剂氯吡格雷阻断 P2Y 12。手术后 24 小时,我们测量了 Sham、CLP 和 CLP + 氯吡格雷组脾脏中 Treg 群体的大小。我们使用人血小板和 T 细胞的共培养物在有或没有抗 CD3/CD28 的情况下研究了体外阻断 P2Y 12的效果。使用 AR-C69931MX 阻止了P2Y 12。与未治疗的脓毒症小鼠相比,用氯吡格雷治疗的脓毒症小鼠的 Treg 种群大小减少。与未经处理的 CLP 小鼠相比,经处理的 CLP 小鼠的血小板和 CD4 + T 细胞的聚集减少。P2Y12拮抗作用会改变血小板在体外影响 T 细胞的方式,这取决于 T 细胞的激活。总之,P2Y 12信号通路的阻断抑制了体内和体外的 Treg 增殖。靶向血小板以控制 Treg 增殖和活性可能是治疗脓毒症的一种有前景的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号