当前位置:
X-MOL 学术
›
Int. J. Energy Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigations on a platinum catalyzed membrane for electrolysis step of copper‐chlorine thermochemical cycle for hydrogen production
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-11-25 , DOI: 10.1002/er.6211 Atindra Mohan Banerjee 1, 2 , Rajini P. Antony 3 , Mrinal R. Pai 1, 2 , Asheesh Kumar 1 , Arvind K. Tripathi 1, 2
International Journal of Energy Research ( IF 4.3 ) Pub Date : 2020-11-25 , DOI: 10.1002/er.6211 Atindra Mohan Banerjee 1, 2 , Rajini P. Antony 3 , Mrinal R. Pai 1, 2 , Asheesh Kumar 1 , Arvind K. Tripathi 1, 2
Affiliation
|
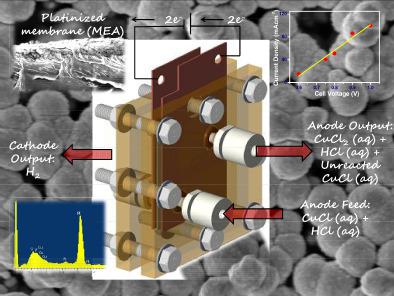
Considering the low temperature demand (~550°C maximum) and high efficiency (~43%), Cu–Cl thermochemical water splitting cycle has immense potential as a futuristic scalable hydrogen generation process. CuCl/HCl electrolysis is the crucial hydrogen generation step in the Cu–Cl thermochemical cycle. The efficiency of this electrolysis process is highly dependent on the performance of the membrane electrode assembly (MEA) employed. To explore the suitability of the platinized membrane for this step, in this study, we have investigated a Pt/Nafion‐117 MEA prepared by in‐situ impregnation‐reduction method for CuCl/HCl electrolysis. A single‐cell PEM‐type electrolyser (4 cm2 active area) consisting of serpentine channels (2 cm × 2 cm) grooved in graphite flow field plates was designed and fabricated to carry out the CuCl/HCl electrolysis. Platinum electrocatalyst was deposited on Nafion‐117 membrane on the cathodic side by reduction of hexachloroplatinic acid (H2PtCl6) with sodium borohydride (NaBH4) by the impregnation‐reduction method which served as the MEA. Monodispersed nanocrystalline Pt particles formed a ~30 μm thick electrocatalyst layer on the membrane. Pt/Nafion‐117 MEA was found to be effective for CuCl /HCl electrolysis. In a PEM‐type electrolyser with Ar‐purged 0.2 M CuCl in ~2 M HCl solution as anolyte flowing at ~ 8.8 mLmin−1, a current density of ~100 mA cm−2 was achieved at a cell voltage of 1 V. However, undesired Cu‐crossover could be noticed from anode to cathode side during operation.
中文翻译:

铜-氯热化学循环制氢电解步骤中铂催化膜的研究
考虑到低温需求(最高约550°C)和高效率(约43%),作为未来可扩展的氢气生产工艺,Cu-Cl热化学水分解循环具有巨大的潜力。CuCl / HCl电解是Cu-Cl热化学循环中至关重要的制氢步骤。该电解过程的效率高度取决于所采用的膜电极组件(MEA)的性能。为了探索镀铂膜在这一步骤中的适用性,在本研究中,我们研究了通过原位浸渍还原方法制备的Pt / Nafion-117 MEA,用于CuCl / HCl电解。单电池PEM型电解槽(4 cm 2设计并制造了由在石墨流场板中开槽的蛇形通道(2厘米×2厘米)组成的有效区域(该区域)以进行CuCl / HCl电解。通过用硼氢化钠(NaBH 4)通过浸渍还原法(MEA )还原六氯铂酸(H 2 PtCl 6),将铂电催化剂沉积在阴极的Nafion-117膜上。单分散的纳米晶Pt颗粒在膜上形成了约30μm厚的电催化剂层。发现Pt / Nafion-117 MEA对CuCl / HCl电解有效。在PEM型电解槽中,使用Ar吹扫的0.2 M CuCl在〜2 M HCl溶液中作为阳极电解液,在〜8.8 mLmin -1下流动,电流密度为〜100 mA cm -2 电池电压为1 V时可达到此目标。但是,在运行过程中,可能会发现阳极到阴极侧发生不希望有的Cu穿越。
更新日期:2020-11-25
中文翻译:

铜-氯热化学循环制氢电解步骤中铂催化膜的研究
考虑到低温需求(最高约550°C)和高效率(约43%),作为未来可扩展的氢气生产工艺,Cu-Cl热化学水分解循环具有巨大的潜力。CuCl / HCl电解是Cu-Cl热化学循环中至关重要的制氢步骤。该电解过程的效率高度取决于所采用的膜电极组件(MEA)的性能。为了探索镀铂膜在这一步骤中的适用性,在本研究中,我们研究了通过原位浸渍还原方法制备的Pt / Nafion-117 MEA,用于CuCl / HCl电解。单电池PEM型电解槽(4 cm 2设计并制造了由在石墨流场板中开槽的蛇形通道(2厘米×2厘米)组成的有效区域(该区域)以进行CuCl / HCl电解。通过用硼氢化钠(NaBH 4)通过浸渍还原法(MEA )还原六氯铂酸(H 2 PtCl 6),将铂电催化剂沉积在阴极的Nafion-117膜上。单分散的纳米晶Pt颗粒在膜上形成了约30μm厚的电催化剂层。发现Pt / Nafion-117 MEA对CuCl / HCl电解有效。在PEM型电解槽中,使用Ar吹扫的0.2 M CuCl在〜2 M HCl溶液中作为阳极电解液,在〜8.8 mLmin -1下流动,电流密度为〜100 mA cm -2 电池电压为1 V时可达到此目标。但是,在运行过程中,可能会发现阳极到阴极侧发生不希望有的Cu穿越。











































 京公网安备 11010802027423号
京公网安备 11010802027423号