当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Ti(OH)OF ⋅ 0.66 H2O in Imidazolium‐based Ionic Liquids
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-11-26 , DOI: 10.1002/open.202000256 Melanie Sieland 1 , Valentine Camus-Genot 1, 2 , Igor Djerdj 3 , Bernd M Smarsly 1, 4
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-11-26 , DOI: 10.1002/open.202000256 Melanie Sieland 1 , Valentine Camus-Genot 1, 2 , Igor Djerdj 3 , Bernd M Smarsly 1, 4
Affiliation
|
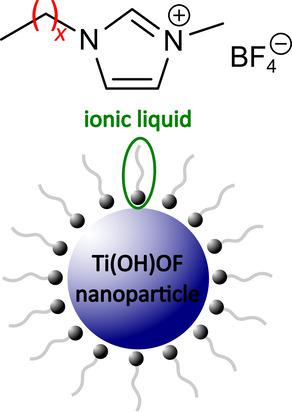
The influence of the cation of imidazolium‐derived ionic liquids (ILs) on a low‐temperature solution‐based synthesis of hexagonal tungsten bronze (HTB) type Ti(OH)OF ⋅ 0.66 H2O and bronze‐type TiO2(B) is investigated. The IL (Cxmim BF4) acts as solvent and also as reaction partner with respect to the decomposition of [BF4]−, releasing F−. In the present study, the chain length of the alkyl chain side groups attached to the imidazolium ring was varied (C2mim BF4 to C10mim BF4), and the obtained solids were analyzed by Powder X‐Ray diffraction (PXRD) followed by Rietveld refinement. As a main finding these analyses indicate a transformation of Ti(OH)OF ⋅ 0.66 H2O into TiO2(B), and upon prolonged reaction time finally also into anatase TiO2. Rietveld analysis suggests that when using ILs with longer alkyl chains, the conversion of Ti(OH)OF ⋅ 0.66 H2O is slower compared to syntheses performed with smaller alkyl chains. Hence, Ti(OH)OF ⋅ 0.66 H2O appears to be metastable and is stabilized by long‐chain ILs serving as surfactant attached to the crystallites’ surface. In this view, the ILs shield the nanoparticles and thus slow down the conversion into the more stable compounds. This confirms previous findings that ILs act as both, solvent and reaction medium in this reaction, thus enabling the synthesis of peculiar Ti‐oxides.
中文翻译:

在咪唑基离子液体中合成 Ti(OH)OF·0.66 H2O
咪唑衍生离子液体(IL)的阳离子对低温溶液合成六方钨青铜(HTB)型Ti(OH)OF ⋅ 0.66 H 2 O和青铜型TiO 2 (B)的影响被调查。IL (C x mim BF 4 ) 充当溶剂,也充当 [BF 4 ] -分解的反应伙伴,释放 F -。在本研究中,咪唑鎓环上连接的烷基链侧基的链长不同(C 2 mim BF 4至C 10 mim BF 4),并通过粉末X射线衍射(PXRD)对所得固体进行分析随后进行 Rietveld 改进。这些分析的主要发现表明,Ti(OH)OF·0.66 H 2 O转化为 TiO 2 (B),并且在延长反应时间后最终也转化为锐钛矿型 TiO 2。Rietveld 分析表明,当使用具有较长烷基链的离子液体时,与使用较小烷基链进行的合成相比,Ti(OH)OF ⋅ 0.66 H 2 O的转化速度较慢。因此,Ti(OH)OF ⋅ 0.66 H 2 O 似乎是亚稳态的,并且通过作为附着在微晶表面的表面活性剂的长链离子液体来稳定。从这个角度来看,离子液体屏蔽了纳米颗粒,从而减缓了向更稳定化合物的转化。这证实了之前的发现,即离子液体在此反应中既充当溶剂又充当反应介质,从而能够合成特殊的钛氧化物。
更新日期:2020-11-26
中文翻译:

在咪唑基离子液体中合成 Ti(OH)OF·0.66 H2O
咪唑衍生离子液体(IL)的阳离子对低温溶液合成六方钨青铜(HTB)型Ti(OH)OF ⋅ 0.66 H 2 O和青铜型TiO 2 (B)的影响被调查。IL (C x mim BF 4 ) 充当溶剂,也充当 [BF 4 ] -分解的反应伙伴,释放 F -。在本研究中,咪唑鎓环上连接的烷基链侧基的链长不同(C 2 mim BF 4至C 10 mim BF 4),并通过粉末X射线衍射(PXRD)对所得固体进行分析随后进行 Rietveld 改进。这些分析的主要发现表明,Ti(OH)OF·0.66 H 2 O转化为 TiO 2 (B),并且在延长反应时间后最终也转化为锐钛矿型 TiO 2。Rietveld 分析表明,当使用具有较长烷基链的离子液体时,与使用较小烷基链进行的合成相比,Ti(OH)OF ⋅ 0.66 H 2 O的转化速度较慢。因此,Ti(OH)OF ⋅ 0.66 H 2 O 似乎是亚稳态的,并且通过作为附着在微晶表面的表面活性剂的长链离子液体来稳定。从这个角度来看,离子液体屏蔽了纳米颗粒,从而减缓了向更稳定化合物的转化。这证实了之前的发现,即离子液体在此反应中既充当溶剂又充当反应介质,从而能够合成特殊的钛氧化物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号