当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses of Acyclic and Macrocyclic Compounds Derived from 9,9‐Diethylfluorene (Part I)
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-11-26 , DOI: 10.1002/open.202000268 Pierre Seidel 1 , Monika Mazik 1
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-11-26 , DOI: 10.1002/open.202000268 Pierre Seidel 1 , Monika Mazik 1
Affiliation
|
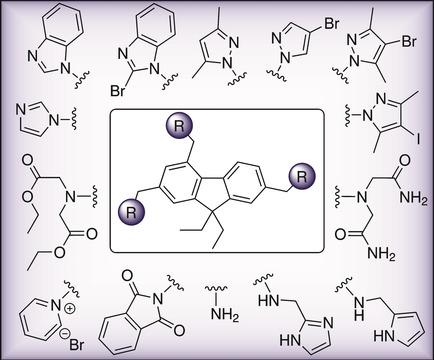
A series of new 9,9‐diethylfluorenes consisting of three side‐arms each bearing a heterocyclic, bis(carboxymethyl)amino, bis(carbamoylmethyl)amino, bis(ethoxycarbonylmethyl)amino or an amino group were prepared on the basis of 2,4,7‐tris(bromomethyl)‐9,9‐diethylfluorene. Imidazolyl, benzimidazolyl, pyrazolyl, pyrrolyl, 1,3‐dioxoisoindolyl and pyridinium groups were taken into account as heterocyclic units, attached to the aromatic skeleton via −CH2−, −CH2NHCH2− or −CH2N=CH− linkers. In addition to the seventeen 2,4,7‐trisubstituted 9,9‐diethylfluorenes, two macrocyclic compounds were prepared on the basis of 2,7‐bis(aminomethyl)‐9,9‐diethylfluorene. The excellent yield of the macrocyclization reaction is worth a special mention. Both the acyclic and the macrocyclic fluorene‐based compounds have, among other things, the potential to act as artificial receptors for different substrates in analogy to the known receptors consisting of a benzene or biphenyl core.
中文翻译:

9,9-二乙基芴衍生的无环和大环化合物的合成(第一部分)
在2,4的基础上制备了一系列由三个侧臂组成的新9,9-二乙基芴,每个侧臂带有杂环、双(羧甲基)氨基、双(氨基甲酰甲基)氨基、双(乙氧基羰基甲基)氨基或氨基。 ,7-三(溴甲基)-9,9-二乙基芴。咪唑基、苯并咪唑基、吡唑基、吡咯基、1,3-二氧异吲哚基和吡啶鎓基团被视为杂环单元,通过-CH 2 -、-CH 2 NHCH 2 -或-CH 2 N=CH-连接基连接到芳香族骨架上。除了十七种2,4,7-三取代的9,9-二乙基芴之外,还制备了两种基于2,7-双(氨基甲基)-9,9-二乙基芴的大环化合物。大环化反应的优异收率值得特别提及。除其他外,无环和大环芴基化合物都具有充当不同底物的人工受体的潜力,类似于由苯或联苯核心组成的已知受体。
更新日期:2020-11-27
中文翻译:

9,9-二乙基芴衍生的无环和大环化合物的合成(第一部分)
在2,4的基础上制备了一系列由三个侧臂组成的新9,9-二乙基芴,每个侧臂带有杂环、双(羧甲基)氨基、双(氨基甲酰甲基)氨基、双(乙氧基羰基甲基)氨基或氨基。 ,7-三(溴甲基)-9,9-二乙基芴。咪唑基、苯并咪唑基、吡唑基、吡咯基、1,3-二氧异吲哚基和吡啶鎓基团被视为杂环单元,通过-CH 2 -、-CH 2 NHCH 2 -或-CH 2 N=CH-连接基连接到芳香族骨架上。除了十七种2,4,7-三取代的9,9-二乙基芴之外,还制备了两种基于2,7-双(氨基甲基)-9,9-二乙基芴的大环化合物。大环化反应的优异收率值得特别提及。除其他外,无环和大环芴基化合物都具有充当不同底物的人工受体的潜力,类似于由苯或联苯核心组成的已知受体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号