当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Dioxide Capture by Chemical Solvents Based on Amino Acids: Absorption and Regeneration
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-12-18 , DOI: 10.1002/ceat.201900562 María Castro 1 , Diego Gómez-Díaz 1 , José M. Navaza 1 , Antonio Rumbo 2
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-12-18 , DOI: 10.1002/ceat.201900562 María Castro 1 , Diego Gómez-Díaz 1 , José M. Navaza 1 , Antonio Rumbo 2
Affiliation
|
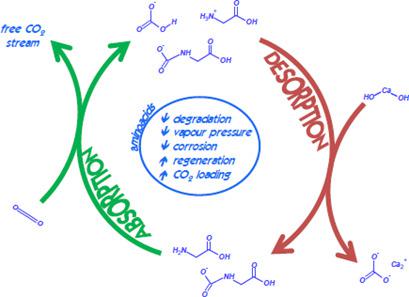
The use of sodium salts of aqueous solutions of different amino acids (glycinate and prolinate) for carbon dioxide (CO2) separation by chemical absorption is analyzed in a bubble‐column reactor under different experimental conditions of solvent concentration and gas flow rate. The differences in absorption rate and reaction mechanism caused by the presence of carboxylic groups in comparison to the case without (monoethanolamine and pirrolidine) are evaluated. Glycinate solvent reaches a similar behavior than monoethanolamine aqueous solutions but with a higher destabilization of carbamate that allows to increase CO2 loading. Prolinate exhibits a similar behavior than pirrolidine at low CO2 loadings. Both solvents show a significant reduction in absorption capacity when stripping regeneration is carried out.
中文翻译:

基于氨基酸的化学溶剂捕集二氧化碳:吸收和再生
在鼓泡塔反应器中,在溶剂浓度和气体流速不同的实验条件下,分析了不同氨基酸(甘氨酸和脯氨酸)水溶液的钠盐通过化学吸收分离二氧化碳(CO 2)的用途。与没有(单乙醇胺和吡咯烷)的情况相比,评估了由羧基的存在引起的吸收速率和反应机理的差异。甘氨酸盐溶剂的行为与单乙醇胺水溶液相似,但是氨基甲酸酯的失稳性更高,可以增加CO 2的负载量。在低CO 2下,脯氨酸盐表现出与吡咯烷类似的行为加载。当进行汽提再生时,两种溶剂均显示出吸收容量的显着降低。
更新日期:2021-01-20
中文翻译:

基于氨基酸的化学溶剂捕集二氧化碳:吸收和再生
在鼓泡塔反应器中,在溶剂浓度和气体流速不同的实验条件下,分析了不同氨基酸(甘氨酸和脯氨酸)水溶液的钠盐通过化学吸收分离二氧化碳(CO 2)的用途。与没有(单乙醇胺和吡咯烷)的情况相比,评估了由羧基的存在引起的吸收速率和反应机理的差异。甘氨酸盐溶剂的行为与单乙醇胺水溶液相似,但是氨基甲酸酯的失稳性更高,可以增加CO 2的负载量。在低CO 2下,脯氨酸盐表现出与吡咯烷类似的行为加载。当进行汽提再生时,两种溶剂均显示出吸收容量的显着降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号