当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Energetics of tetradentate N2O2 Schiff bases containing different alkyldiimine brigdes
Thermochimica Acta ( IF 3.1 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.tca.2020.178817 Ana L.R. Silva , Jorge M. Gonçalves , Victor M.F. Morais , Maria D.M.C. Ribeiro da Silva
Thermochimica Acta ( IF 3.1 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.tca.2020.178817 Ana L.R. Silva , Jorge M. Gonçalves , Victor M.F. Morais , Maria D.M.C. Ribeiro da Silva
|
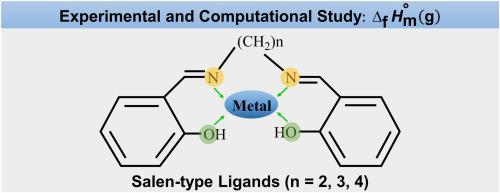
Abstract This work is part of a comprehensive study of the thermal properties of several molecules of tetradentate Schiff bases, obtained as condensation products of salicylaldehyde with alkyldiamines. Herein, we report an experimental thermochemical study of SALPN (N,N´-bis(salicylaldehydo)propylenediimine) and a computational thermochemical study of SALEN (N,N´-bis(salicylaldehydo)dimethylenediimine), SALPN and SALBUTEN (N,N´-bis(salicylaldehydo)tetramethylenediimine) ligands. The standard (p° =0.1 MPa) molar enthalpy of formation of crystalline SALPN, at T = 298.15 K, was determined using the static-bomb calorimetry technique. Also, the enthalpy of fusion of this ligand has been determined by differential scanning calorimetry. Additionally, using quantum chemical calculations at the CCSD(T) level of theory, we have calculated the gas-phase standard molar enthalpies of formation of three Schiff base ligands, SALEN, SALPN and SALBUTEN. Moreover, a computational study of the molecular structures of the ligands has been carried out.
中文翻译:

含有不同烷基二亚胺桥键的四齿 N2O2 席夫碱的能量学
摘要 这项工作是对几个四齿席夫碱分子的热性质进行综合研究的一部分,这些分子是水杨醛与烷基二胺的缩合产物。在此,我们报告了 SALPN(N,N´-双(水杨醛)丙烯二亚胺)的实验热化学研究和 SALEN(N,N´-双(水杨醛)二亚甲基亚胺)、SALPN 和 SALBUTEN(N,N´ -双(水杨醛)四亚甲基亚胺)配体。在 T = 298.15 K 时,使用静态弹量热法测定标准 (p° = 0.1 MPa) 结晶 SALPN 的摩尔形成焓。此外,该配体的融合焓已通过差示扫描量热法测定。此外,在 CCSD(T) 理论水平上使用量子化学计算,我们已经计算了三种席夫碱配体 SALEN、SALPN 和 SALBUTEN 的气相标准摩尔形成焓。此外,还对配体的分子结构进行了计算研究。
更新日期:2021-01-01
中文翻译:

含有不同烷基二亚胺桥键的四齿 N2O2 席夫碱的能量学
摘要 这项工作是对几个四齿席夫碱分子的热性质进行综合研究的一部分,这些分子是水杨醛与烷基二胺的缩合产物。在此,我们报告了 SALPN(N,N´-双(水杨醛)丙烯二亚胺)的实验热化学研究和 SALEN(N,N´-双(水杨醛)二亚甲基亚胺)、SALPN 和 SALBUTEN(N,N´ -双(水杨醛)四亚甲基亚胺)配体。在 T = 298.15 K 时,使用静态弹量热法测定标准 (p° = 0.1 MPa) 结晶 SALPN 的摩尔形成焓。此外,该配体的融合焓已通过差示扫描量热法测定。此外,在 CCSD(T) 理论水平上使用量子化学计算,我们已经计算了三种席夫碱配体 SALEN、SALPN 和 SALBUTEN 的气相标准摩尔形成焓。此外,还对配体的分子结构进行了计算研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号