Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-11-26 , DOI: 10.1016/j.jcat.2020.11.012 Liwen Wang , Yida Xu , Teng Chen , Dali Wei , Xuefeng Guo , Luming Peng , Nianhua Xue , Yan Zhu , Mengning Ding , Weiping Ding
|
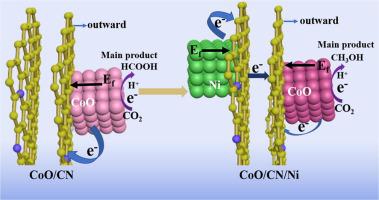
Methanol is one of the most desirable C1 products in the electrocatalytic CO2 reduction reaction (CO2RR). The intricate nature of the reaction pathway involving six-electron transfer, however, makes the process highly challenging. In this work, we report a ternary heterostructural catalyst CoO/CN/Ni, with cobalt as catalytic centers supported on N-doped carbon with underlying nickel, which presents a remarkably promoted Faraday efficiency (70.7%) and current density (10.6 mA/cm2) towards methanol, compared with CoO/CN. It appears that the nickel underneath plays an essential role by donating electrons to outer CN layer and thus changes the electronic state of surface and then of the cobalt species which act as catalytic centers. Notably, the unique catalyst shows not only high electrocatalytic activity and selectivity to CH3OH, but also suppression of H2 formation and well-preserved catalytic performance during the long-term test of reaction. Simultaneously, isotropic labeling and electrochemical experiment prove the carbon source.
中文翻译:

三元异质结构CoO / CN / Ni催化剂,用于促进CO 2电还原为甲醇
甲醇是电催化CO 2还原反应(CO 2 RR)中最理想的C1产品之一。然而,涉及六电子转移的反应路径的复杂性质使该过程极具挑战性。在这项工作中,我们报告了一种三元异质结构催化剂CoO / CN / Ni,其中钴作为催化中心负载在掺有底层镍的N掺杂碳上,从而显着提高了法拉第效率(70.7%)和电流密度(10.6 mA / cm)2与CoO / CN相比)。似乎下面的镍通过向外部CN层提供电子,从而改变了表面的电子状态,进而改变了充当催化中心的钴物种的电子状态,起着至关重要的作用。值得注意的是,该独特的催化剂不仅显示出高的电催化活性和对CH 3 OH的选择性,而且还抑制了H 2的形成并在长期的反应测试中保留了良好的催化性能。同时,各向同性标记和电化学实验证明了碳源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号