Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-11-26 , DOI: 10.1016/j.jcat.2020.11.017 Rubén Rizo , Arno Bergmann , Janis Timoshenko , Fabian Scholten , Clara Rettenmaier , Hyo Sang Jeon , Yen-Ting Chen , Aram Yoon , Alexander Bagger , Jan Rossmeisl , Beatriz Roldan Cuenya
|
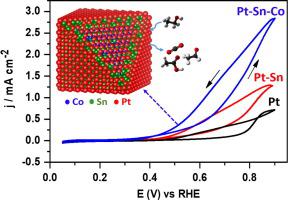
Direct ethanol fuel cells are among the most promising clean electrochemical power sources. Nevertheless, the high cost and low efficiency of the Pt-based catalysts hinder their commercialization. Here, Pt-Sn-Co nanocubes with a Pt- and Sn-rich shell show improved performance towards the electrochemical ethanol oxidation reaction (EOR). Mechanistic and structural insights were obtained by synergistically combining different in situ and operando spectro-electrochemical techniques, including electrochemical mass spectrometry, X-ray photoelectron spectroscopy and X-ray absorption spectroscopy. In particular, electrochemical conditioning and EOR were found to induce Sn leaching from the core and shell, leading to electrochemically-accessible Pt sites adjacent to partially-oxidized Sn sites on a Pt3Co-like core. The increased activity of the Pt-Sn-Co nanocubes was assigned to the formation of a higher amount of C1 (CO2) and C2 (acetic acid/acetaldehyde) products during EOR as well as to their high ability to remove adsorbed CO from the Pt surface when compared to similarly-sized cubic Pt-Sn or Pt NPs. Beneficial strain and ligand effects are combined here through a catalyst design resulting in adjacent Pt and Sn sites at the overlayer on top of a Pt3Co alloy core.
中文翻译:

Pt-Sn-Co纳米立方体作为乙醇电氧化的高活性催化剂
直接乙醇燃料电池是最有前途的清洁电化学电源之一。然而,Pt基催化剂的高成本和低效率阻碍了它们的商业化。在这里,具有富含Pt和Sn的壳的Pt-Sn-Co纳米立方体对电化学乙醇氧化反应(EOR)的性能有所提高。机械和结构方面的见解是通过将不同的原位和操作性协同结合而获得的光谱电化学技术,包括电化学质谱法,X射线光电子能谱和X射线吸收光谱。特别是,发现电化学调节和EOR诱导了Sn从核和壳中的浸出,从而导致与Pt 3 Co状核上部分氧化的Sn部位相邻的电化学可及的Pt部位。Pt-Sn-Co纳米立方体活性的提高被归因于更高数量的C 1(CO 2)和C 2的形成。(乙酸/乙醛)产品在EOR期间以及与类似尺寸的立方Pt-Sn或Pt NPs相比具有从Pt表面去除吸附的CO的高能力。有益的应变和配体效应在此处通过催化剂设计结合在一起,从而在Pt 3 Co合金芯顶部的覆盖层上产生相邻的Pt和Sn位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号