Immunity ( IF 25.5 ) Pub Date : 2020-11-26 , DOI: 10.1016/j.immuni.2020.11.017 Joana P Bernardes 1 , Neha Mishra 1 , Florian Tran 2 , Thomas Bahmer 3 , Lena Best 4 , Johanna I Blase 1 , Dora Bordoni 1 , Jeanette Franzenburg 5 , Ulf Geisen 6 , Jonathan Josephs-Spaulding 4 , Philipp Köhler 7 , Axel Künstner 8 , Elisa Rosati 1 , Anna C Aschenbrenner 9 , Petra Bacher 10 , Nathan Baran 1 , Teide Boysen 1 , Burkhard Brandt 11 , Niklas Bruse 12 , Jonathan Dörr 6 , Andreas Dräger 13 , Gunnar Elke 14 , David Ellinghaus 1 , Julia Fischer 15 , Michael Forster 1 , Andre Franke 1 , Sören Franzenburg 1 , Norbert Frey 16 , Anette Friedrichs 3 , Janina Fuß 1 , Andreas Glück 3 , Jacob Hamm 1 , Finn Hinrichsen 1 , Marc P Hoeppner 1 , Simon Imm 1 , Ralf Junker 11 , Sina Kaiser 6 , Ying H Kan 1 , Rainer Knoll 17 , Christoph Lange 18 , Georg Laue 1 , Clemens Lier 11 , Matthias Lindner 14 , Georgios Marinos 4 , Robert Markewitz 11 , Jacob Nattermann 19 , Rainer Noth 3 , Peter Pickkers 12 , Klaus F Rabe 20 , Alina Renz 13 , Christoph Röcken 21 , Jan Rupp 22 , Annika Schaffarzyk 6 , Alexander Scheffold 23 , Jonas Schulte-Schrepping 24 , Domagoj Schunk 25 , Dirk Skowasch 26 , Thomas Ulas 27 , Klaus-Peter Wandinger 11 , Michael Wittig 1 , Johannes Zimmermann 4 , Hauke Busch 28 , Bimba F Hoyer 6 , Christoph Kaleta 4 , Jan Heyckendorf 16 , Matthijs Kox 29 , Jan Rybniker 15 , Stefan Schreiber 2 , Joachim L Schultze 27 , Philip Rosenstiel 1 , ,
|
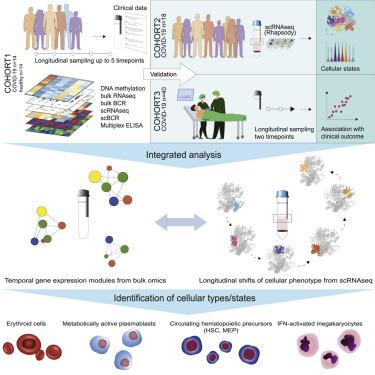
Temporal resolution of cellular features associated with a severe COVID-19 disease trajectory is needed for understanding skewed immune responses and defining predictors of outcome. Here, we performed a longitudinal multi-omics study using a two-center cohort of 14 patients. We analyzed the bulk transcriptome, bulk DNA methylome, and single-cell transcriptome (>358,000 cells, including BCR profiles) of peripheral blood samples harvested from up to 5 time points. Validation was performed in two independent cohorts of COVID-19 patients. Severe COVID-19 was characterized by an increase of proliferating, metabolically hyperactive plasmablasts. Coinciding with critical illness, we also identified an expansion of interferon-activated circulating megakaryocytes and increased erythropoiesis with features of hypoxic signaling. Megakaryocyte- and erythroid-cell-derived co-expression modules were predictive of fatal disease outcome. The study demonstrates broad cellular effects of SARS-CoV-2 infection beyond adaptive immune cells and provides an entry point toward developing biomarkers and targeted treatments of patients with COVID-19.
中文翻译:

纵向多组学分析将巨核细胞、红细胞和浆母细胞的反应确定为严重 COVID-19 的标志
需要对与严重 COVID-19 疾病轨迹相关的细胞特征进行时间解析,以了解偏差的免疫反应和定义结果的预测因子。在这里,我们使用 14 名患者的两个中心队列进行了纵向多组学研究。我们分析了从多达 5 个时间点采集的外周血样本的大量转录组、大量 DNA 甲基化组和单细胞转录组(>358,000 个细胞,包括 BCR 谱)。在两个独立的 COVID-19 患者队列中进行了验证。重症 COVID-19 的特征是增殖、代谢过度活跃的浆母细胞增多。与危重病相吻合,我们还发现干扰素激活的循环巨核细胞增多,红细胞生成增加,具有缺氧信号传导的特征。巨核细胞和红细胞衍生的共表达模块可预测致命的疾病结果。该研究证明了 SARS-CoV-2 感染对适应性免疫细胞以外的广泛细胞影响,并为开发生物标志物和 COVID-19 患者的靶向治疗提供了一个切入点。









































 京公网安备 11010802027423号
京公网安备 11010802027423号