当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantiospecific on-water bromination: a mild and efficient protocol for the preparation of alkyl bromides
Green Chemistry ( IF 9.3 ) Pub Date : 2020-11-12 , DOI: 10.1039/d0gc02855j Francesco Alletto 1, 2, 3, 4, 5 , Mauro F. A. Adamo 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020-11-12 , DOI: 10.1039/d0gc02855j Francesco Alletto 1, 2, 3, 4, 5 , Mauro F. A. Adamo 1, 2, 3, 4, 5
Affiliation
|
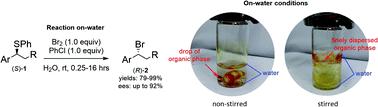
Herein we report the first example of an on-water enantiospecific synthesis of alkyl bromides. This procedure allowed the conversion of secondary activated alkyl sulphides to benzylic alkyl bromides, which were obtained in 80–99% yields. The reaction carried out on enantio-pure sulphides provided the corresponding bromides in high yields and enantioselectivity (up to 92% ee; 94% es) at room temperature. The on-water conditions reduced significantly the reaction times compared to similar procedures run in organic media. The condition identified made use of no solvent, required no temperature control and produced a smooth organic phase easily separated for further synthetic use on a multigram-scale without the need for any organic extraction. Therefore, the present constitutes the most operationally simple and environmentally benign approach to a class of much sought organic intermediates.
中文翻译:

对映体特异性水上溴化:温和而有效的方案,用于制备烷基溴化物
在本文中,我们报告了烷基溴的水对映体特异性合成的第一个例子。该程序允许将次级活化烷基硫化物转化为苄基烷基溴化物,其收率为80-99%。在室温下,对映体纯的硫化物进行的反应以高收率和对映体选择性(最高92%ee; 94%es)提供了相应的溴化物。与在有机介质中运行的类似程序相比,水上条件显着减少了反应时间。所确定的条件不使用溶剂,不需要控制温度,并且产生了易于分离的光滑有机相,可用于数克规模的进一步合成用途,而无需任何有机萃取。因此,
更新日期:2020-11-25
中文翻译:

对映体特异性水上溴化:温和而有效的方案,用于制备烷基溴化物
在本文中,我们报告了烷基溴的水对映体特异性合成的第一个例子。该程序允许将次级活化烷基硫化物转化为苄基烷基溴化物,其收率为80-99%。在室温下,对映体纯的硫化物进行的反应以高收率和对映体选择性(最高92%ee; 94%es)提供了相应的溴化物。与在有机介质中运行的类似程序相比,水上条件显着减少了反应时间。所确定的条件不使用溶剂,不需要控制温度,并且产生了易于分离的光滑有机相,可用于数克规模的进一步合成用途,而无需任何有机萃取。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号