当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the Protein Functions and Absorption Wavelengths of Microbial Rhodopsins
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jpcb.0c08910 Masaki Tsujimura 1 , Hiroshi Ishikita 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jpcb.0c08910 Masaki Tsujimura 1 , Hiroshi Ishikita 1, 2
Affiliation
|
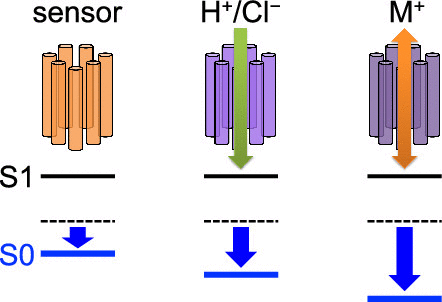
Using a quantum mechanical/molecular mechanical approach, the absorption wavelength of the retinal Schiff base was calculated based on 13 microbial rhodopsin crystal structures. The results showed that the protein electrostatic environment decreases the absorption wavelength significantly in the cation-conducting rhodopsin but only slightly in the sensory rhodopsin. Among the microbial rhodopsins with different functions, the differences in the absorption wavelengths are caused by differences in the arrangement of the charged residues at the retinal Schiff base binding moiety, namely, one or two counterions at the three common positions. Among the microbial rhodopsins with similar functions, the differences in the polar residues at the retinal Schiff base binding site are responsible for the differences in the absorption wavelengths. Counterions contribute to an absorption wavelength shift of 50–120 nm, whereas polar groups contribute to a shift of up to ∼10 nm. It seems likely that protein function is directly associated with the absorption wavelength in microbial rhodopsins.
中文翻译:

深入了解微生物视紫红质的蛋白质功能和吸收波长
使用量子力学/分子力学方法,基于13种微生物视紫红质晶体结构计算了视网膜席夫碱的吸收波长。结果表明,蛋白质静电环境显着降低了阳离子传导性视紫红质中的吸收波长,而在感官视紫红质中仅略微降低了吸收波长。在具有不同功能的微生物视紫红质中,吸收波长的差异是由于视网膜席夫碱结合部分处带电残基(即,三个共同位置处的一个或两个抗衡离子)的排列差异引起的。在具有相似功能的微生物视紫红质中,视网膜席夫碱结合位点上极性残基的差异是造成吸收波长差异的原因。抗衡离子的吸收波长偏移为50–120 nm,而极性基团的吸收波长偏移可达〜10 nm。蛋白质功能似乎与微生物视紫红质的吸收波长直接相关。
更新日期:2020-12-31
中文翻译:

深入了解微生物视紫红质的蛋白质功能和吸收波长
使用量子力学/分子力学方法,基于13种微生物视紫红质晶体结构计算了视网膜席夫碱的吸收波长。结果表明,蛋白质静电环境显着降低了阳离子传导性视紫红质中的吸收波长,而在感官视紫红质中仅略微降低了吸收波长。在具有不同功能的微生物视紫红质中,吸收波长的差异是由于视网膜席夫碱结合部分处带电残基(即,三个共同位置处的一个或两个抗衡离子)的排列差异引起的。在具有相似功能的微生物视紫红质中,视网膜席夫碱结合位点上极性残基的差异是造成吸收波长差异的原因。抗衡离子的吸收波长偏移为50–120 nm,而极性基团的吸收波长偏移可达〜10 nm。蛋白质功能似乎与微生物视紫红质的吸收波长直接相关。










































 京公网安备 11010802027423号
京公网安备 11010802027423号