当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Micellar Solution Behavior of Cetylpyridinium Surfactants in 2-Propanol–Water Mixed Media at Different Temperatures
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jced.0c00734 Yumnam Gyani Devi 1 , Jackson Gurung 1 , Ajmal Koya Pulikkal 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jced.0c00734 Yumnam Gyani Devi 1 , Jackson Gurung 1 , Ajmal Koya Pulikkal 1
Affiliation
|
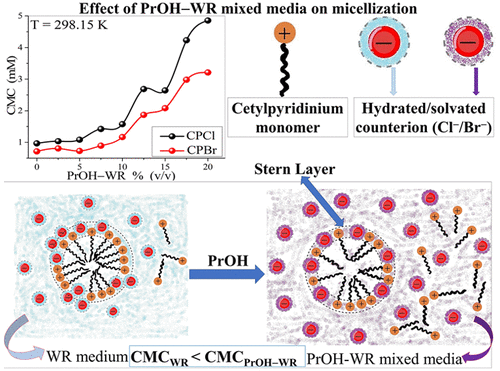
Self-assembly of surfactant systems in organic solvent–water mixed media is relevant in several industrial and pharmaceutical applications. This article emphasizes the role of 2-propanol (PrOH) as a cosolvent in the micellization of cetylpyridinium chloride (CPCl) and cetylpyridinium bromide (CPBr) surfactants at different temperatures (293.15–308.15 K) studied through the conductometric technique. The results show an enhancement in CMC values for both the surfactants with an increase in volume percentage (vol %) of PrOH (from 0 up to 20 vol %) as well as temperature. The CPBr shows distinct behavior in the lower composition of PrOH–WR (0 and 2.5%) at a higher temperature (308.15 K). The rise in the CMC values depends on lowering of permittivity of the mixed media caused by change in compositions of PrOH–WR mixed media. A similar effect is also seen on the degree of counterion dissociation (α) values with an exception to a few compositions. The increase in α values is found to be relatively less for CPBr as compared to CPCl. Temperature dependency of CMC values has been used to calculate thermodynamic parameters such as the change in Gibbs energy (ΔGm0), enthalpy (ΔHm0), and entropy (ΔSm0) of micellization, and their values were used to analyze the effect of various compositions of PrOH–WR mixed media. The ΔGm0 values indicate that micellization is spontaneous and becomes less favorable with the inclusion of PrOH. The values of ΔHm0(<0) and ΔSm0(>0) affirm that the process of micellization is entropically driven within the scope of the studied temperature. Overall results have been analyzed based on the interactions between the solute and solvent, counterions and aggregates, and solvation/desolvation of counterions.
中文翻译:

十六烷基吡啶表面活性剂在2-丙二醇-水混合介质中在不同温度下的胶束溶液行为
表面活性剂体系在有机溶剂-水混合介质中的自组装与几种工业和制药应用有关。本文重点介绍了通过电导技术研究的在不同温度(293.15–308.15 K)下十六烷基氯化吡啶鎓(CPCl)和十六烷基溴化吡啶鎓(CPBr)表面活性剂胶束化过程中,2-丙醇(PrOH)作为助溶剂的作用。结果显示两种表面活性剂的CMC值均随PrOH的体积百分比(vol%)(从0至20vol%)和温度的增加而增加。CPBr在较高温度(308.15 K)下,在较低的PrOH-WR组成(0和2.5%)中表现出明显的行为。CMC值的上升取决于PrOH-WR混合介质组成变化导致混合介质介电常数降低。除少数成分外,对平衡离子解离度(α)值的影响也相似。与CPCP1相比,发现CPBr的α值的增加相对较小。CMC值的温度依赖性已用于计算热力学参数,例如吉布斯能量(Δ胶束化的G m 0),焓(ΔH m 0)和熵(ΔS m 0)及其值被用来分析各种成分的PrOH-WR混合介质的影响。该Δ ģ米0值表明胶束是自发的和变得一起列入PrOH中的不太有利的。Δ的值ħ中号0(<0)和Δ小号米0(> 0)确认胶束化过程是在研究温度范围内熵驱动的。根据溶质与溶剂,抗衡离子和聚集体之间的相互作用以及抗衡离子的溶剂化/去溶剂化作用,对总体结果进行了分析。
更新日期:2021-01-14
中文翻译:

十六烷基吡啶表面活性剂在2-丙二醇-水混合介质中在不同温度下的胶束溶液行为
表面活性剂体系在有机溶剂-水混合介质中的自组装与几种工业和制药应用有关。本文重点介绍了通过电导技术研究的在不同温度(293.15–308.15 K)下十六烷基氯化吡啶鎓(CPCl)和十六烷基溴化吡啶鎓(CPBr)表面活性剂胶束化过程中,2-丙醇(PrOH)作为助溶剂的作用。结果显示两种表面活性剂的CMC值均随PrOH的体积百分比(vol%)(从0至20vol%)和温度的增加而增加。CPBr在较高温度(308.15 K)下,在较低的PrOH-WR组成(0和2.5%)中表现出明显的行为。CMC值的上升取决于PrOH-WR混合介质组成变化导致混合介质介电常数降低。除少数成分外,对平衡离子解离度(α)值的影响也相似。与CPCP1相比,发现CPBr的α值的增加相对较小。CMC值的温度依赖性已用于计算热力学参数,例如吉布斯能量(Δ胶束化的G m 0),焓(ΔH m 0)和熵(ΔS m 0)及其值被用来分析各种成分的PrOH-WR混合介质的影响。该Δ ģ米0值表明胶束是自发的和变得一起列入PrOH中的不太有利的。Δ的值ħ中号0(<0)和Δ小号米0(> 0)确认胶束化过程是在研究温度范围内熵驱动的。根据溶质与溶剂,抗衡离子和聚集体之间的相互作用以及抗衡离子的溶剂化/去溶剂化作用,对总体结果进行了分析。









































 京公网安备 11010802027423号
京公网安备 11010802027423号