当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Measurement of Carbon Dioxide Solubility in Aqueous N-Methyldiethanolamine + 2-(2-Aminoethylamino) Ethanol + Sulfolane and Diethanolamine + 2-(2-Aminoethylamino) Ethanol + Sulfolane Hybrid Solvents at Various Temperatures and High Pressure
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jced.0c00739 Ehsan Asadi 1 , Ali Haghtalab 1 , Habib Allah Shirazizadeh 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jced.0c00739 Ehsan Asadi 1 , Ali Haghtalab 1 , Habib Allah Shirazizadeh 1
Affiliation
|
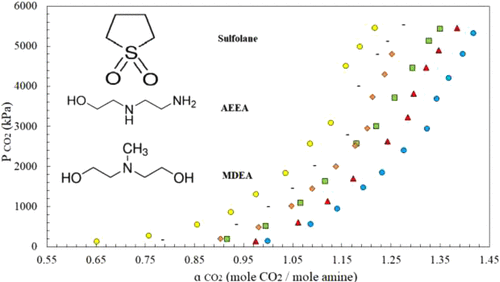
The sets of vapor–liquid equilibrium (VLE) data for CO2 solubility in a combination of (N-methyldiethanolamine (MDEA) + 2-(2-aminoethylamino) ethanol (AEEA)) and (diethanolamine (DEA) + AEEA) as a chemical solvent blended with sulfolane as a physical solvent are acquired at 313.15–343.15 K within the CO2 partial pressure range up to 5600 kPa. The measurements are fulfilled for two different hybrid solvents of MDEA + AEEA + sulfolane with the weight compositions of (20–10–10) wt % and (20–10–20) wt % and a mixed solvent of DEA + AEEA + sulfolane with (20–10–10) wt %. It is deduced that CO2 loading was enhanced by substituting the MDEA with DEA, diminishing the temperature, and raising the pressure. Besides, increasing the sulfolane concentration in the aqueous system of MDEA + AEEA + sulfolane leads to enrichment in the effectiveness of the solution in CO2 absorption in the high loading region. In contrast, at low pressures, it adversely affects the acid gas loading. Moreover, it was displayed that at the same total mole of alkanolamines and sulfolane, the CO2 solubilities of AEEA + sulfolane and AEEA + MDEA + sulfolane are higher than those of AEEA + MDEA + sulfolane and MDEA + sulfolane, respectively. Therefore, it could be claimed that AEEA presents a better performance in CO2 capturing than MDEA. Besides, it was demonstrated that AEEA + sulfolane represents a higher capability in CO2 elimination than DEA + AEEA + sulfolane. Thus, the ability of DEA to remove CO2 is lesser than AEEA. Altogether, the degree of alkanolamines’ selectivity toward CO2 is found as AEEA > MDEA > DEA.
中文翻译:

N-甲基二乙醇胺+ 2-(2-氨基乙基氨基)乙醇+环丁砜和二乙醇胺+ 2-(2-氨基乙基氨基)乙醇+环丁砜杂化溶剂在不同温度和高压下二氧化碳溶解度的实验测量
在(N-甲基二乙醇胺(MDEA)+ 2-(2-氨基乙基氨基)乙醇(AEEA))和(二乙醇胺(DEA)+ AEEA)的组合下,CO 2溶解度的气液平衡(VLE)数据集在313.15–343.15 K下,在最高5600 kPa的CO 2分压范围内,获得了与环丁砜作为物理溶剂混合的化学溶剂。对于两种不同的MDEA + AEEA +环丁砜混合溶剂,其重量组成分别为(20–10–10)wt%和(20–10–20–20)wt%,以及DEA + AEEA +环丁砜与(20–10–10)wt%。据推断,CO 2通过用DEA代替MDEA,降低温度并提高压力来增加负荷。此外,增加MDEA + AEEA +环丁砜水溶液体系中环丁砜浓度会导致溶液在高负载区域吸收CO 2的有效性增加。相反,在低压下,它会对酸性气体的负载产生不利影响。此外,显示出在相同的链烷醇胺和环丁砜的总摩尔数下,AEEA +环丁砜和AEEA + MDEA +环丁砜的CO 2溶解度分别高于AEEA + MDEA +环丁砜和MDEA +环丁砜的CO 2溶解度。因此,可以说AEEA在CO 2中表现出更好的性能。比MDEA捕获。此外,已证明AEEA +环丁砜比DEA + AEEA +环丁砜具有更高的CO 2消除能力。因此,DEA去除CO 2的能力小于AEEA。总而言之,发现链烷醇胺对CO 2的选择性程度为AEEA> MDEA> DEA。
更新日期:2021-01-14
中文翻译:

N-甲基二乙醇胺+ 2-(2-氨基乙基氨基)乙醇+环丁砜和二乙醇胺+ 2-(2-氨基乙基氨基)乙醇+环丁砜杂化溶剂在不同温度和高压下二氧化碳溶解度的实验测量
在(N-甲基二乙醇胺(MDEA)+ 2-(2-氨基乙基氨基)乙醇(AEEA))和(二乙醇胺(DEA)+ AEEA)的组合下,CO 2溶解度的气液平衡(VLE)数据集在313.15–343.15 K下,在最高5600 kPa的CO 2分压范围内,获得了与环丁砜作为物理溶剂混合的化学溶剂。对于两种不同的MDEA + AEEA +环丁砜混合溶剂,其重量组成分别为(20–10–10)wt%和(20–10–20–20)wt%,以及DEA + AEEA +环丁砜与(20–10–10)wt%。据推断,CO 2通过用DEA代替MDEA,降低温度并提高压力来增加负荷。此外,增加MDEA + AEEA +环丁砜水溶液体系中环丁砜浓度会导致溶液在高负载区域吸收CO 2的有效性增加。相反,在低压下,它会对酸性气体的负载产生不利影响。此外,显示出在相同的链烷醇胺和环丁砜的总摩尔数下,AEEA +环丁砜和AEEA + MDEA +环丁砜的CO 2溶解度分别高于AEEA + MDEA +环丁砜和MDEA +环丁砜的CO 2溶解度。因此,可以说AEEA在CO 2中表现出更好的性能。比MDEA捕获。此外,已证明AEEA +环丁砜比DEA + AEEA +环丁砜具有更高的CO 2消除能力。因此,DEA去除CO 2的能力小于AEEA。总而言之,发现链烷醇胺对CO 2的选择性程度为AEEA> MDEA> DEA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号