当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Study of Physical Properties and CO2 Solubility of Ammonium and Sulfonium Ionic Liquids in Mixture with Glutaronitrile
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jced.0c00740 Bao Kou Xiong 1 , Malaurie Paillot 1 , Mérièm Anouti 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.jced.0c00740 Bao Kou Xiong 1 , Malaurie Paillot 1 , Mérièm Anouti 1
Affiliation
|
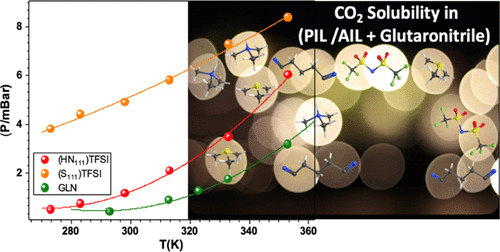
In this work, we present a comparative study of physical properties and CO2 solubility of two ionic liquids (ILs), protic trimethylammonium bis[(trifluoromethyl)sulfonyl]imide and aprotic trimethylsulfonium bis[(trifluoromethyl) sulfonyl]imide in mixture with glutaronitrile (GLN). The effect of temperature on density and viscosity of mixtures was studied and discussed in terms of intra- and intermolecular interactions. Applying Glasser’s theory, standard molar entropy, S0, and the crystal energy, UPOT, of the IL/GLN mixtures were estimated, showing a positive contribution of ILs to these quantities. This positive contribution to the entropy variation by the addition of an IL is because of the fact that it destroys GLN–GLN interactions, replacing them with new cation–GLN and anion–GLN interactions. The deconstructing effect of S111 is greater because there are no strong S111–GLN interactions, unlike the ammonium cation which can establish H-bonds with C≡N groups. The CO2 solubilities in both (IL/GLN) mixtures expressed by CO2 molar fractions, xCO2, or the Henry constant KH/MPa showed that the [S111][TFSI]/GLN solubilizes more CO2 than the [HN111][TFSI]/GLN throughout the temperature range. The solubility of CO2 is sensitive to the cation nature and temperature. The sulfonium cation promotes CO2 solubility because it is less cluttered than the ammonium cation unlike HN111, which is the hydrogen-binding seat (NH–GLN) with GLN. Finally, the thermodynamic parameters such as the standard enthalpy ΔdissH0, the free enthalpy ΔdissG0, and the entropy ΔdissS0 of CO2 dissolution were calculated. They showed that the dissolution of CO2 did not depend on the molecular organization (small variation in entropy) but that it is linked to the intermolecular interactions in the IL/GLN mixture, as shown by the difference in ΔmixH.
中文翻译:

铵和S离子液体与戊二腈混合物的物理性质和CO 2溶解度的比较研究
在这项工作中,我们对两种离子液体(ILs)质子化三甲基铵双[((三氟甲基)磺酰基]酰亚胺和非质子化三甲基ulf双[(三氟甲基)磺酰基]酰亚胺与戊二腈混合物的物理性质和CO 2溶解度进行了比较研究。GLN)。根据分子内和分子间的相互作用研究和讨论了温度对混合物密度和粘度的影响。应用Glasser理论,标准摩尔熵S 0和晶体能量U POT估计了IL / GLN混合物的,显示IL对这些数量有积极贡献。添加IL对熵变的积极贡献是因为它破坏了GLN-GLN相互作用,并用新的阳离子-GLN和阴离子-GLN相互作用取代了它们。S 111的解构作用更大,因为不存在强烈的S 111 -GLN相互作用,这不同于铵阳离子可以与C≡N基团建立H键。两种(IL / GLN)混合物中的CO 2溶解度均以CO 2摩尔分数x CO 2或亨利常数K H / MPa表示,表明[S 111在整个温度范围内,与[HN 111 ] [TFSI] / GLN相比,] [TFSI] / GLN可溶解更多的CO 2。CO 2的溶解度对阳离子的性质和温度敏感。H阳离子与HN 111不同,它比铵阳离子更杂乱,因此可促进CO 2溶解,而HN 111是GLN的氢结合位点(NH-GLN)。最后,给出了CO 2的标准焓Δdiss H 0,自由焓Δdiss G 0和熵Δdiss S 0等热力学参数。计算溶出度。他们指出,CO的溶解2不依赖于所述分子组织(在熵小的变化),但它与在IL / GLN混合物中的分子间相互作用,如由Δ的差混合ħ。
更新日期:2021-01-14
中文翻译:

铵和S离子液体与戊二腈混合物的物理性质和CO 2溶解度的比较研究
在这项工作中,我们对两种离子液体(ILs)质子化三甲基铵双[((三氟甲基)磺酰基]酰亚胺和非质子化三甲基ulf双[(三氟甲基)磺酰基]酰亚胺与戊二腈混合物的物理性质和CO 2溶解度进行了比较研究。GLN)。根据分子内和分子间的相互作用研究和讨论了温度对混合物密度和粘度的影响。应用Glasser理论,标准摩尔熵S 0和晶体能量U POT估计了IL / GLN混合物的,显示IL对这些数量有积极贡献。添加IL对熵变的积极贡献是因为它破坏了GLN-GLN相互作用,并用新的阳离子-GLN和阴离子-GLN相互作用取代了它们。S 111的解构作用更大,因为不存在强烈的S 111 -GLN相互作用,这不同于铵阳离子可以与C≡N基团建立H键。两种(IL / GLN)混合物中的CO 2溶解度均以CO 2摩尔分数x CO 2或亨利常数K H / MPa表示,表明[S 111在整个温度范围内,与[HN 111 ] [TFSI] / GLN相比,] [TFSI] / GLN可溶解更多的CO 2。CO 2的溶解度对阳离子的性质和温度敏感。H阳离子与HN 111不同,它比铵阳离子更杂乱,因此可促进CO 2溶解,而HN 111是GLN的氢结合位点(NH-GLN)。最后,给出了CO 2的标准焓Δdiss H 0,自由焓Δdiss G 0和熵Δdiss S 0等热力学参数。计算溶出度。他们指出,CO的溶解2不依赖于所述分子组织(在熵小的变化),但它与在IL / GLN混合物中的分子间相互作用,如由Δ的差混合ħ。









































 京公网安备 11010802027423号
京公网安备 11010802027423号