当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Investigation of the Preferred Binding Modes of N2O in Group 8 Metal Complexes
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.inorgchem.0c02903 Kylie Fields 1 , Brian M. Barngrover 1 , J. Brannon Gary 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.inorgchem.0c02903 Kylie Fields 1 , Brian M. Barngrover 1 , J. Brannon Gary 1
Affiliation
|
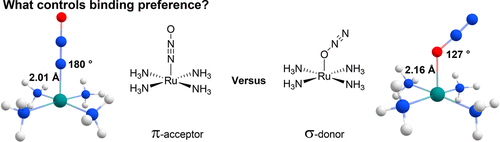
Nitrous oxide (N2O) is a potentially important oxidant for green chemistry applications but thus far has shown limited examples as a ligand for transition metal complexes. Given the lack of reported N2O complexes, density functional theory was utilized to study the potential binding effects in multiple group 8 metal complexes. N2O is found to be a very weakly π-accepting ligand (approximately 1/3 as effective as CO). With the weak π-accepting character, the N2O is predicted to be bound through the nitrogen atom in a linear geometry. In all calculated ruthenium and osmium complexes, the nitrogen bound mode of binding is preferred. Only by introduction of a very weak π-donor metal (such as iron) can the N2O be found to slightly prefer binding through the oxygen atom in a purely σ-donor fashion.
中文翻译:

第8族金属配合物中N 2 O优选结合方式的计算研究
一氧化二氮(N 2 O)是绿色化学应用中潜在的重要氧化剂,但到目前为止,作为过渡金属配合物的配体,已显示出有限的例子。由于缺乏报道的N 2 O配合物,因此采用密度泛函理论研究了多种8族金属配合物中的潜在结合作用。发现N 2 O是一种非常弱的π接受配体(大约是CO的1/3)。由于π接受性弱,因此可以预测N 2 O以线性几何形状通过氮原子结合。在所有计算出的钌和配合物中,氮结合的结合方式是优选的。N 2只能通过引入非常弱的π供体金属(例如铁)来实现人们发现稍微偏爱纯粹以σ供体方式通过氧原子结合。
更新日期:2020-12-21
中文翻译:

第8族金属配合物中N 2 O优选结合方式的计算研究
一氧化二氮(N 2 O)是绿色化学应用中潜在的重要氧化剂,但到目前为止,作为过渡金属配合物的配体,已显示出有限的例子。由于缺乏报道的N 2 O配合物,因此采用密度泛函理论研究了多种8族金属配合物中的潜在结合作用。发现N 2 O是一种非常弱的π接受配体(大约是CO的1/3)。由于π接受性弱,因此可以预测N 2 O以线性几何形状通过氮原子结合。在所有计算出的钌和配合物中,氮结合的结合方式是优选的。N 2只能通过引入非常弱的π供体金属(例如铁)来实现人们发现稍微偏爱纯粹以σ供体方式通过氧原子结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号