当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Curli-Mediated Self-Assembly of a Fibrous Protein Scaffold for Hydroxyapatite Mineralization
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-25 , DOI: 10.1021/acssynbio.0c00415 Zahra Abdali 1 , Masoud Aminzare 1 , Xiaodan Zhu 1 , Elizabeth DeBenedictis 2 , Oliver Xie 1 , Sinan Keten 2 , Noémie-Manuelle Dorval Courchesne 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-11-25 , DOI: 10.1021/acssynbio.0c00415 Zahra Abdali 1 , Masoud Aminzare 1 , Xiaodan Zhu 1 , Elizabeth DeBenedictis 2 , Oliver Xie 1 , Sinan Keten 2 , Noémie-Manuelle Dorval Courchesne 1
Affiliation
|
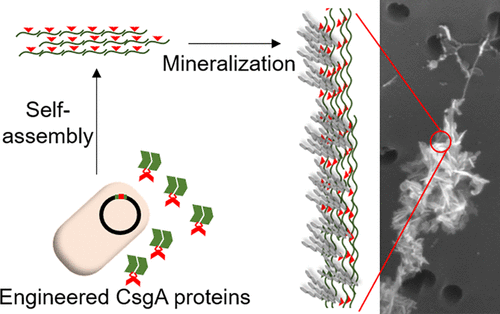
Nanostructures formed by self-assembled peptides have been increasingly exploited as functional materials for a wide variety of applications, from biotechnology to energy. However, it is sometimes challenging to assemble free short peptides into functional supramolecular structures, since not all peptides have the ability to self-assemble. Here, we report a self-assembly mechanism for short functional peptides that we derived from a class of fiber-forming amyloid proteins called curli. CsgA, the major subunit of curli fibers, is a self-assembling β-helical subunit composed of five pseudorepeats (R1–R5). We first deleted the internal repeats (R2, R3, R4), known to be less essential for the aggregation of CsgA monomers into fibers, forming a truncated CsgA variant (R1/R5). As a proof-of-concept to introduce functionality in the fibers, we then genetically substituted the internal repeats by a hydroxyapatite (HAP)-binding peptide, resulting in a R1/HAP/R5 construct. Our method thus utilizes the R1/R5-driven self-assembly mechanism to assemble the HAP-binding peptide and form hydrogel-like materials in macroscopic quantities suitable for biomineralization. We confirmed the expression and fibrillar morphology of the truncated and HAP-containing curli-like amyloid fibers. X-ray diffraction and TEM showed the functionality of the HAP-binding peptide for mineralization and formation of nanocrystalline HAP. Overall, we show that fusion to the R1 and R5 repeats of CsgA enables the self-assembly of functional peptides into micron long fibers. Further, the mineral-templating ability that the R1/HAP/R5 fibers possesses opens up broader applications for curli proteins in the tissue engineering and biomaterials fields.
中文翻译:

纤维蛋白支架的Curli介导的自组装,用于羟基磷灰石矿化
自组装肽形成的纳米结构已被越来越多地用作功能材料,用于从生物技术到能源的各种应用。然而,有时将自由的短肽组装成功能性超分子结构具有挑战性,因为并非所有的肽都具有自组装能力。在这里,我们报告了一种短功能肽的自组装机制,该短功能肽来自一类称为curli的纤维形成淀粉样蛋白。CsgA是卷曲纤维的主要亚基,是由五个假重复序列(R1-R5)组成的自组装β螺旋亚基。我们首先删除了内部重复序列(R2,R3,R4),这些重复序列对于将CsgA单体聚集到纤维中的重要性较小,从而形成了截短的CsgA变异体(R1 / R5)。作为在光纤中引入功能的概念证明,然后,我们用羟基磷灰石(HAP)结合肽基因取代内部重复序列,从而形成R1 / HAP / R5构建体。因此,我们的方法利用R1 / R5驱动的自组装机制组装HAP结合肽,并形成适合生物矿化的宏观量的水凝胶状材料。我们证实了截短和含HAP的curli样淀粉样纤维的表达和原纤维形态。X射线衍射和TEM显示HAP结合肽用于矿化和形成纳米晶体HAP的功能。总体而言,我们表明融合到CsgA的R1和R5重复序列可以使功能性肽自组装成微米长的纤维。进一步,
更新日期:2020-12-18
中文翻译:

纤维蛋白支架的Curli介导的自组装,用于羟基磷灰石矿化
自组装肽形成的纳米结构已被越来越多地用作功能材料,用于从生物技术到能源的各种应用。然而,有时将自由的短肽组装成功能性超分子结构具有挑战性,因为并非所有的肽都具有自组装能力。在这里,我们报告了一种短功能肽的自组装机制,该短功能肽来自一类称为curli的纤维形成淀粉样蛋白。CsgA是卷曲纤维的主要亚基,是由五个假重复序列(R1-R5)组成的自组装β螺旋亚基。我们首先删除了内部重复序列(R2,R3,R4),这些重复序列对于将CsgA单体聚集到纤维中的重要性较小,从而形成了截短的CsgA变异体(R1 / R5)。作为在光纤中引入功能的概念证明,然后,我们用羟基磷灰石(HAP)结合肽基因取代内部重复序列,从而形成R1 / HAP / R5构建体。因此,我们的方法利用R1 / R5驱动的自组装机制组装HAP结合肽,并形成适合生物矿化的宏观量的水凝胶状材料。我们证实了截短和含HAP的curli样淀粉样纤维的表达和原纤维形态。X射线衍射和TEM显示HAP结合肽用于矿化和形成纳米晶体HAP的功能。总体而言,我们表明融合到CsgA的R1和R5重复序列可以使功能性肽自组装成微米长的纤维。进一步,











































 京公网安备 11010802027423号
京公网安备 11010802027423号