Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Propelled and Near-Infrared-Phototaxic Photosynthetic Bacteria as Photothermal Agents for Hypoxia-Targeted Cancer Therapy
ACS Nano ( IF 15.8 ) Pub Date : 2020-11-25 , DOI: 10.1021/acsnano.0c08068 Pengli Zheng 1 , Miao Fan 1, 2 , Huifang Liu 3 , Yinghua Zhang 1 , Xinyue Dai 1, 2 , Hang Li 1 , Xiaohan Zhou 2 , Shiqi Hu 4, 5 , Xinjian Yang 1, 2 , Yi Jin 2 , Na Yu 6 , Shutao Guo 6 , Jinchao Zhang 2 , Xing-Jie Liang 7 , Ke Cheng 4, 5 , Zhenhua Li 1, 2, 4, 5
ACS Nano ( IF 15.8 ) Pub Date : 2020-11-25 , DOI: 10.1021/acsnano.0c08068 Pengli Zheng 1 , Miao Fan 1, 2 , Huifang Liu 3 , Yinghua Zhang 1 , Xinyue Dai 1, 2 , Hang Li 1 , Xiaohan Zhou 2 , Shiqi Hu 4, 5 , Xinjian Yang 1, 2 , Yi Jin 2 , Na Yu 6 , Shutao Guo 6 , Jinchao Zhang 2 , Xing-Jie Liang 7 , Ke Cheng 4, 5 , Zhenhua Li 1, 2, 4, 5
Affiliation
|
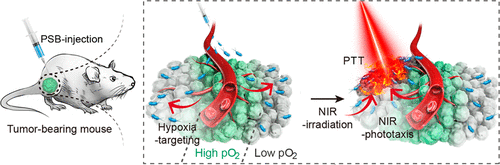
Hypoxia can increase the resistance of tumor cells to radiotherapy and chemotherapy. However, the dense extracellular matrix, high interstitial fluid pressure, and irregular blood supply often serve as physical barriers to inhibit penetration of drugs or nanodrugs across tumor blood microvessels into hypoxic regions. Therefore, it is of great significance and highly desirable to improve the efficiency of hypoxia-targeted therapy. In this work, living photosynthetic bacteria (PSB) are utilized as hypoxia-targeted carriers for hypoxic tumor therapy due to their near-infrared (NIR) chemotaxis and their physiological characteristics as facultative aerobes. More interestingly, we discovered that PSB can serve as a kind of photothermal agent to generate heat through nonradiative relaxation pathways due to their strong photoabsorption in the NIR region. Therefore, PSB integrate the properties of hypoxia targeting and photothermal therapeutic agents in an “all-in-one” manner, and no postmodification is needed to achieve hypoxia-targeted cancer therapy. Moreover, as natural bacteria, noncytotoxic PSB were found to enhance immune response that induced the infiltration of cytotoxicity T lymphocyte. Our results indicate PSB specifically accumulate in hypoxic tumor regions, and they show a high efficiency in the elimination of cancer cells. This proof of concept may provide a smart therapeutic system in the field of hypoxia-targeted photothermal therapeutic platforms.
中文翻译:

自走式和近红外光合作用光合细菌作为低氧靶向癌症治疗的光热剂
缺氧可增加肿瘤细胞对放疗和化疗的抵抗力。然而,致密的细胞外基质,高的组织液压力和不规则的血液供应通常充当物理屏障,以抑制药物或纳米药物跨过肿瘤血液微血管渗入缺氧区域。因此,提高缺氧靶向治疗的效率具有重要意义。在这项工作中,由于它们的近红外(NIR)趋化性和它们作为兼性需氧菌的生理特性,活的光合细菌(PSB)被用作低氧肿瘤治疗的靶向低氧载体。更有趣的是,我们发现PSB可以用作一种光热剂,因为它们在NIR区域具有很强的光吸收能力,因此可以通过非辐射弛豫途径产生热量。因此,PSB以“多合一”方式整合了缺氧靶向和光热治疗剂的特性,并且无需进行后修饰即可实现针对缺氧的癌症治疗。此外,作为天然细菌,发现无细胞毒性的PSB增强了诱导细胞毒性T淋巴细胞浸润的免疫反应。我们的结果表明PSB专门积聚在缺氧肿瘤区域中,并且在消除癌细胞方面显示出很高的效率。该概念证明可在针对缺氧的光热治疗平台领域提供智能治疗系统。发现非细胞毒性的PSB增强了诱导细胞毒性T淋巴细胞浸润的免疫反应。我们的结果表明PSB专门积聚在缺氧肿瘤区域中,并且在消除癌细胞方面显示出很高的效率。该概念证明可在针对缺氧的光热治疗平台领域提供智能治疗系统。发现非细胞毒性的PSB增强了诱导细胞毒性T淋巴细胞浸润的免疫反应。我们的结果表明PSB专门积聚在缺氧肿瘤区域中,并且在消除癌细胞方面显示出很高的效率。该概念证明可在针对缺氧的光热治疗平台领域提供智能治疗系统。
更新日期:2021-01-26
中文翻译:

自走式和近红外光合作用光合细菌作为低氧靶向癌症治疗的光热剂
缺氧可增加肿瘤细胞对放疗和化疗的抵抗力。然而,致密的细胞外基质,高的组织液压力和不规则的血液供应通常充当物理屏障,以抑制药物或纳米药物跨过肿瘤血液微血管渗入缺氧区域。因此,提高缺氧靶向治疗的效率具有重要意义。在这项工作中,由于它们的近红外(NIR)趋化性和它们作为兼性需氧菌的生理特性,活的光合细菌(PSB)被用作低氧肿瘤治疗的靶向低氧载体。更有趣的是,我们发现PSB可以用作一种光热剂,因为它们在NIR区域具有很强的光吸收能力,因此可以通过非辐射弛豫途径产生热量。因此,PSB以“多合一”方式整合了缺氧靶向和光热治疗剂的特性,并且无需进行后修饰即可实现针对缺氧的癌症治疗。此外,作为天然细菌,发现无细胞毒性的PSB增强了诱导细胞毒性T淋巴细胞浸润的免疫反应。我们的结果表明PSB专门积聚在缺氧肿瘤区域中,并且在消除癌细胞方面显示出很高的效率。该概念证明可在针对缺氧的光热治疗平台领域提供智能治疗系统。发现非细胞毒性的PSB增强了诱导细胞毒性T淋巴细胞浸润的免疫反应。我们的结果表明PSB专门积聚在缺氧肿瘤区域中,并且在消除癌细胞方面显示出很高的效率。该概念证明可在针对缺氧的光热治疗平台领域提供智能治疗系统。发现非细胞毒性的PSB增强了诱导细胞毒性T淋巴细胞浸润的免疫反应。我们的结果表明PSB专门积聚在缺氧肿瘤区域中,并且在消除癌细胞方面显示出很高的效率。该概念证明可在针对缺氧的光热治疗平台领域提供智能治疗系统。











































 京公网安备 11010802027423号
京公网安备 11010802027423号