当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Convenient method for the synthesis of some novel chiral methyl 2‐(2‐oxo‐2H‐benzo[e][1,3]oxazin‐3(4H)‐yl)propanoate derivatives and biological evaluation of their antioxidant, cytotoxic, and molecular docking properties
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-11-25 , DOI: 10.1002/jhet.4196 Sivakumar Matam 1 , Prabakaran Kaliyan 1 , Loganathan Selvaraj 1 , Seenivasa Perumal Muthu 1 , Bharathi Priya Lohanathan 2 , Vijaya Padma Viswanadhan 2 , Himesh Makala 3 , Ulaganathan Venkatasubramanian 3
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-11-25 , DOI: 10.1002/jhet.4196 Sivakumar Matam 1 , Prabakaran Kaliyan 1 , Loganathan Selvaraj 1 , Seenivasa Perumal Muthu 1 , Bharathi Priya Lohanathan 2 , Vijaya Padma Viswanadhan 2 , Himesh Makala 3 , Ulaganathan Venkatasubramanian 3
Affiliation
|
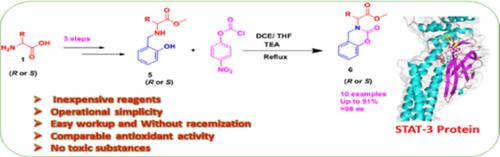
Ten chiral methyl 2‐(2‐oxo‐2H‐benzo[e][1,3]oxazin‐3(4H)‐yl)propanoate derivatives 6a‐6j have been synthesized from optically pure amino methyl phenol 5 and 4‐nitrophenyl chloroformate. These derivatives 6a‐6j are characterized by 1H NMR, 13C NMR, FT‐IR, and HRMS spectral techniques. Optical purity of these derivatives was confirmed by chiral HPLC method. Ten synthesized ester derivatives 6a‐6j were screened for their in vitro antioxidant activity. Among the compounds 6b‐d and 6h‐j have exhibited comparable antioxidant activity with ascorbic acid as a standard. Compounds 6a and 6e‐g have shown moderate antioxidant activity. Further, the in vitro cytotoxicity of these compounds were studied through MTT cell proliferation assay in addition the effect on LDH leakage and NO release. Among the derivatives, 6j showed extremely best activity and the IC50 value (12.54 ± 0.71 μM) is very close to doxorubicin (7.2 ± 0.58 μM) as a standard. Compounds 6b, 6h, and 6i showed better inhibition next to compound 6j on the viability of HepG2 cells with an IC50 value (μM) of 56.02 ± 1.4, 41.76 ± 0.58, and 38.17 ± 0.34, respectively. Also, molecular docking studies have been carried out with STAT‐3 (PDB ID: 1BG1) and BCL‐2 (PDB ID: 4AQ3) proteins against the four active compounds 6b, 6h, 6i, and 6j. The binding energies of the tested compounds were in the range of −7.76 to −8.41 kcal/mol, which is very close to doxorubicin (−8.53 kcal/mol) as a standard. These molecular docking results are in good agreement with the in vitro studies.
中文翻译:

合成一些新型手性2-(2-氧代-2H-苯并[e] [1,3]恶嗪-3(4H)-基)丙酸酯衍生物的简便方法及其抗氧化剂,细胞毒性和分子生物学评估对接属性
从光学纯的氨基甲基苯酚5和4-硝基苯基氯甲酸酯合成了十种手性2-(2-oxo-2H-苯并[e] [1,3]恶嗪-3(4H)-基)丙酸酯甲基酯衍生物6a-6j。这些衍生物6a-6j的特征在于1 H NMR,13 C NMR,FT-IR和HRMS光谱技术。通过手性HPLC法确认了这些衍生物的光学纯度。筛选了十种合成的酯衍生物6a-6j的体外抗氧化活性。在化合物6b-d和6h-j中,抗坏血酸的抗氧化活性与标准抗坏血酸相当。化合物6a和6e‐g表现出适度的抗氧化活性。此外,还通过MTT细胞增殖试验研究了这些化合物的体外细胞毒性,此外还对LDH泄漏和NO释放有影响。在这些衍生物中,6j表现出极好的活性,并且标准的IC 50值(12.54±0.71μM)非常接近阿霉素(7.2±0.58μM)。化合物6b,6h和6i对具有IC 50的HepG2细胞生存力的抑制作用优于化合物6j值(μM)分别为56.02±1.4、41.76±0.58和38.17±0.34。此外,已经针对三种活性化合物6b,6h,6i和6j对STAT-3(PDB ID:1BG1)和BCL-2(PDB ID:4AQ3)蛋白进行了分子对接研究。被测化合物的结合能在-7.76至-8.41 kcal / mol的范围内,非常接近于标准的阿霉素(-8.53 kcal / mol)。这些分子对接的结果与体外研究非常吻合。
更新日期:2020-11-25
中文翻译:

合成一些新型手性2-(2-氧代-2H-苯并[e] [1,3]恶嗪-3(4H)-基)丙酸酯衍生物的简便方法及其抗氧化剂,细胞毒性和分子生物学评估对接属性
从光学纯的氨基甲基苯酚5和4-硝基苯基氯甲酸酯合成了十种手性2-(2-oxo-2H-苯并[e] [1,3]恶嗪-3(4H)-基)丙酸酯甲基酯衍生物6a-6j。这些衍生物6a-6j的特征在于1 H NMR,13 C NMR,FT-IR和HRMS光谱技术。通过手性HPLC法确认了这些衍生物的光学纯度。筛选了十种合成的酯衍生物6a-6j的体外抗氧化活性。在化合物6b-d和6h-j中,抗坏血酸的抗氧化活性与标准抗坏血酸相当。化合物6a和6e‐g表现出适度的抗氧化活性。此外,还通过MTT细胞增殖试验研究了这些化合物的体外细胞毒性,此外还对LDH泄漏和NO释放有影响。在这些衍生物中,6j表现出极好的活性,并且标准的IC 50值(12.54±0.71μM)非常接近阿霉素(7.2±0.58μM)。化合物6b,6h和6i对具有IC 50的HepG2细胞生存力的抑制作用优于化合物6j值(μM)分别为56.02±1.4、41.76±0.58和38.17±0.34。此外,已经针对三种活性化合物6b,6h,6i和6j对STAT-3(PDB ID:1BG1)和BCL-2(PDB ID:4AQ3)蛋白进行了分子对接研究。被测化合物的结合能在-7.76至-8.41 kcal / mol的范围内,非常接近于标准的阿霉素(-8.53 kcal / mol)。这些分子对接的结果与体外研究非常吻合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号