当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and in vitro Characterization of Tricyclic Benzodiazepine Derivatives as Potent and Selective Anti‐leukemic Agents
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-12-30 , DOI: 10.1002/cbdv.202000733 Adam Mieczkowski 1 , Tomasz Frączyk 1, 2 , Mateusz Psurski 3 , Patrycja Wińska 4 , Paweł Siedlecki 1 , Monika Dziełak 1 , Damian Trzybiński 5 , Marcin Wilczek 6 , Maciej Bagiński 1, 6 , Bartosz Bieszczad 1 , Krzysztof Woźniak 5
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-12-30 , DOI: 10.1002/cbdv.202000733 Adam Mieczkowski 1 , Tomasz Frączyk 1, 2 , Mateusz Psurski 3 , Patrycja Wińska 4 , Paweł Siedlecki 1 , Monika Dziełak 1 , Damian Trzybiński 5 , Marcin Wilczek 6 , Maciej Bagiński 1, 6 , Bartosz Bieszczad 1 , Krzysztof Woźniak 5
Affiliation
|
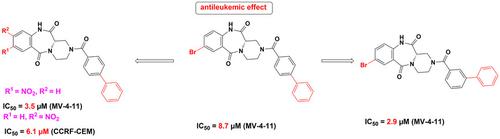
Currently available chemotherapeutic treatments for blood cancers (leukemia) usually have strong side effects. More selective, efficient, and less toxic anticancer agents are needed. We synthesized seven, new, optically pure (12aS)‐1,3,4,12a‐tetrahydropyrazino[2,1‐c][1,4],12(2H,11H)‐dione derivatives and examined their cytotoxicity towards eight cancer cell lines, including urinary bladder (TCC‐SUP, UM‐UC‐3, KU‐19‐9), colon (LoVo), and breast (MCF‐7, MDA‐MB‐231) cancer representatives, as well as two leukemic cell lines (MV‐4‐11, CCRF‐CEM) and normal murine fibroblasts (Balb/3T3) as reference cell line. Three of the seven newly‐obtained compounds ((12aS)‐8‐bromo‐2‐(3‐phenylbenzoyl)‐1,3,4,12a‐tetrahydropyrazino[2,1‐c][1,4],12(2H,11H)‐dione, (12aS)‐8,9‐dimethoxy‐2‐(4‐phenylbenzoyl)‐1,3,4,12a‐tetrahydropyrazino[2,1‐c][1,4],12(2H,11H)‐dione and (12aS)‐8‐nitro‐2‐(4‐phenylbenzoyl)‐1,3,4,12a‐tetrahydropyrazino[2,1‐c][1,4],12(2H,11H)‐dione, showed enhanced activity and selectivity toward the leukemic MV‐4‐11 cell lines when compared to our previously reported compounds, with IC50 values in the range of 2.9–5.6 μM. Additionally, (12aS)‐9‐nitro‐2‐(4‐phenylbenzoyl)‐1,3,4,12a‐tetrahydropyrazino[2,1‐c][1,4],12(2H,11H)‐dione exhibited a strong cytotoxic effect against the leukemic CCRF‐CEM (IC50=6.1 μM) and MV‐4‐11 (IC50=11.0 μM) cell lines, a moderate cytotoxic effect toward other tumor lines (IC50=31.8–55.0 μM) and very weak cytotoxic effect toward the Balb/3T3 reference cell lines. Selected compounds were further evaluated for their potential to induce apoptotic cell death in MV‐4‐11 cells by measuring caspase‐3 activity. We also established the crystal structure of three products and investigated the effect of 22 derivatives of 1,3,4,12a‐tetrahydropyrazino[2,1‐c][1,4],12(2H,11H)‐dione on the activity of the cancer‐associated enzyme autotaxin. All compounds proved to be weak inhibitors of autotaxin, although some (R) and (S) enantiomers had Ki values of 10–19 μM. The obtained results showed that the tested compounds exhibited a selective antileukemic effect, which appeared not to be related directly to autotaxin. Molecular targets responsible for this effect remain to be identified. The newly obtained compounds can be used in the search for new, selective anticancer therapies.
中文翻译:

三环苯二氮卓衍生物作为强效和选择性抗白血病药物的设计和体外表征
目前可用的血癌(白血病)化疗药物通常具有很强的副作用。需要更具选择性、更有效且毒性更小的抗癌剂。我们合成了七种新型光学纯 (12aS)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H,11H)-二酮衍生物,并检测了它们对八种癌症的细胞毒性细胞系,包括膀胱(TCC-SUP、UM-UC-3、KU-19-9)、结肠(LoVo)和乳腺癌(MCF-7、MDA-MB-231)癌症代表,以及两种白血病细胞系(MV-4-11,CCRF-CEM)和正常鼠成纤维细胞(Balb/3T3)作为参考细胞系。七个新获得的化合物中的三个 ((12aS)-8-bromo-2-(3-phenylbenzoyl)-1,3,4,12a-tetrahydropyrazino[2,1-c][1,4],12(2H ,11H)-二酮, (12aS)-8,9-二甲氧基-2-(4-苯基苯甲酰基)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H, 11H)-二酮和 (12aS)-8-硝基-2-(4-苯基苯甲酰基)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H,11H)-与我们之前报道的化合物相比,dione 对白血病 MV-4-11 细胞系显示出增强的活性和选择性,IC50 值在 2.9-5.6 μM 的范围内。此外,(12aS)-9-硝基-2-(4-苯基苯甲酰基)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H,11H)-二酮表现出对白血病 CCRF-CEM (IC50=6.1 μM) 和 MV-4-11 (IC50=11.0 μM) 细胞系有强细胞毒作用,对其他肿瘤系有中等细胞毒作用 (IC50=31.8-55.0 μM) 和非常弱的细胞毒作用对 Balb/3T3 参考细胞系的影响。通过测量 caspase-3 活性,进一步评估所选化合物在 MV-4-11 细胞中诱导细胞凋亡的潜力。我们还建立了三种产物的晶体结构,并研究了 1,3,4,12a-四氢吡嗪并 [2,1-c][1,4],12(2H,11H)-二酮的 22 种衍生物对活性的影响癌症相关酶自分泌运动因子的研究。尽管一些 (R) 和 (S) 对映异构体的 Ki 值为 10–19 μM,但所有化合物都被证明是自分泌运动因子的弱抑制剂。获得的结果表明,受试化合物表现出选择性抗白血病作用,这似乎与自分泌运动因子没有直接关系。负责这种效应的分子靶点仍有待确定。新获得的化合物可用于寻找新的、选择性的抗癌疗法。尽管一些 (R) 和 (S) 对映异构体的 Ki 值为 10–19 μM,但所有化合物都被证明是自分泌运动因子的弱抑制剂。获得的结果表明,受试化合物表现出选择性抗白血病作用,这似乎与自分泌运动因子没有直接关系。负责这种效应的分子靶点仍有待确定。新获得的化合物可用于寻找新的、选择性的抗癌疗法。尽管一些 (R) 和 (S) 对映异构体的 Ki 值为 10–19 μM,但所有化合物都被证明是自分泌运动因子的弱抑制剂。获得的结果表明,受试化合物表现出选择性抗白血病作用,这似乎与自分泌运动因子没有直接关系。负责这种效应的分子靶点仍有待确定。新获得的化合物可用于寻找新的、选择性的抗癌疗法。
更新日期:2020-12-30
中文翻译:

三环苯二氮卓衍生物作为强效和选择性抗白血病药物的设计和体外表征
目前可用的血癌(白血病)化疗药物通常具有很强的副作用。需要更具选择性、更有效且毒性更小的抗癌剂。我们合成了七种新型光学纯 (12aS)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H,11H)-二酮衍生物,并检测了它们对八种癌症的细胞毒性细胞系,包括膀胱(TCC-SUP、UM-UC-3、KU-19-9)、结肠(LoVo)和乳腺癌(MCF-7、MDA-MB-231)癌症代表,以及两种白血病细胞系(MV-4-11,CCRF-CEM)和正常鼠成纤维细胞(Balb/3T3)作为参考细胞系。七个新获得的化合物中的三个 ((12aS)-8-bromo-2-(3-phenylbenzoyl)-1,3,4,12a-tetrahydropyrazino[2,1-c][1,4],12(2H ,11H)-二酮, (12aS)-8,9-二甲氧基-2-(4-苯基苯甲酰基)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H, 11H)-二酮和 (12aS)-8-硝基-2-(4-苯基苯甲酰基)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H,11H)-与我们之前报道的化合物相比,dione 对白血病 MV-4-11 细胞系显示出增强的活性和选择性,IC50 值在 2.9-5.6 μM 的范围内。此外,(12aS)-9-硝基-2-(4-苯基苯甲酰基)-1,3,4,12a-四氢吡嗪并[2,1-c][1,4],12(2H,11H)-二酮表现出对白血病 CCRF-CEM (IC50=6.1 μM) 和 MV-4-11 (IC50=11.0 μM) 细胞系有强细胞毒作用,对其他肿瘤系有中等细胞毒作用 (IC50=31.8-55.0 μM) 和非常弱的细胞毒作用对 Balb/3T3 参考细胞系的影响。通过测量 caspase-3 活性,进一步评估所选化合物在 MV-4-11 细胞中诱导细胞凋亡的潜力。我们还建立了三种产物的晶体结构,并研究了 1,3,4,12a-四氢吡嗪并 [2,1-c][1,4],12(2H,11H)-二酮的 22 种衍生物对活性的影响癌症相关酶自分泌运动因子的研究。尽管一些 (R) 和 (S) 对映异构体的 Ki 值为 10–19 μM,但所有化合物都被证明是自分泌运动因子的弱抑制剂。获得的结果表明,受试化合物表现出选择性抗白血病作用,这似乎与自分泌运动因子没有直接关系。负责这种效应的分子靶点仍有待确定。新获得的化合物可用于寻找新的、选择性的抗癌疗法。尽管一些 (R) 和 (S) 对映异构体的 Ki 值为 10–19 μM,但所有化合物都被证明是自分泌运动因子的弱抑制剂。获得的结果表明,受试化合物表现出选择性抗白血病作用,这似乎与自分泌运动因子没有直接关系。负责这种效应的分子靶点仍有待确定。新获得的化合物可用于寻找新的、选择性的抗癌疗法。尽管一些 (R) 和 (S) 对映异构体的 Ki 值为 10–19 μM,但所有化合物都被证明是自分泌运动因子的弱抑制剂。获得的结果表明,受试化合物表现出选择性抗白血病作用,这似乎与自分泌运动因子没有直接关系。负责这种效应的分子靶点仍有待确定。新获得的化合物可用于寻找新的、选择性的抗癌疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号