当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cephalosporin Prodrug Inhibitors Overcome Metallo‐β‐Lactamase Driven Antibiotic Resistance
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-11-25 , DOI: 10.1002/chem.202004694 Matthijs J van Haren 1 , Kamaleddin H M E Tehrani 1 , Ioli Kotsogianni 1 , Nicola Wade 1 , Nora C Brüchle 1 , Vida Mashayekhi 2 , Nathaniel I Martin 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-11-25 , DOI: 10.1002/chem.202004694 Matthijs J van Haren 1 , Kamaleddin H M E Tehrani 1 , Ioli Kotsogianni 1 , Nicola Wade 1 , Nora C Brüchle 1 , Vida Mashayekhi 2 , Nathaniel I Martin 1
Affiliation
|
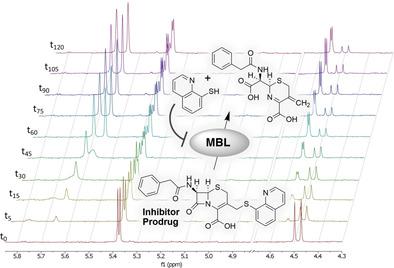
The increasing prevalence of metallo‐β‐lactamase (MBL)‐expressing bacteria presents a worrying trend in antibiotic resistance. MBLs rely on active site zinc ions for their hydrolytic activity and the pursuit of MBL‐inhibitors has therefore involved the investigation of zinc chelators. To ensure that such chelators specifically target MBLs, a series of cephalosporin prodrugs of two potent zinc‐binders: dipicolinic acid (DPA) and 8‐thioquinoline (8‐TQ) was prepared. Although both DPA and 8‐TQ bind free zinc very tightly (Kd values in the low nm range), the corresponding cephalosporin conjugates do not. The cephalosporin conjugates are efficiently hydrolyzed by MBLs to release DPA or 8‐TQ, as confirmed by using both NMR and LC‐MS studies. Notably, the cephalosporin prodrugs of DPA and 8‐TQ show potent inhibitory activity against NDM, VIM, and IMP classes of MBLs and display potent synergy with meropenem against MBL‐expressing clinical isolates of K. pneumoniae and E. coli.
中文翻译:

头孢菌素前药抑制剂克服金属-β-内酰胺酶驱动的抗生素耐药性
表达金属-β-内酰胺酶(MBL)的细菌的日益流行呈现出令人担忧的抗生素耐药性趋势。MBL 的水解活性依赖于活性位点锌离子,因此对 MBL 抑制剂的研究涉及锌螯合剂的研究。为了确保此类螯合剂特异性针对 MBL,制备了两种有效的锌结合剂:吡啶二甲酸 (DPA) 和 8-硫代喹啉 (8-TQ) 的一系列头孢菌素前药。尽管 DPA 和 8-TQ 都非常紧密地结合游离锌(K d值在低 nm范围内),但相应的头孢菌素缀合物则不然。NMR 和 LC-MS 研究证实,头孢菌素缀合物可被 MBL 有效水解,释放 DPA 或 8-TQ。值得注意的是,DPA 和 8-TQ 的头孢菌素前药对 NDM、VIM 和 IMP 类 MBL 表现出有效的抑制活性,并与美罗培南对表达 MBL 的肺炎克雷伯菌和大肠杆菌临床分离株表现出有效的协同作用。
更新日期:2020-11-25
中文翻译:

头孢菌素前药抑制剂克服金属-β-内酰胺酶驱动的抗生素耐药性
表达金属-β-内酰胺酶(MBL)的细菌的日益流行呈现出令人担忧的抗生素耐药性趋势。MBL 的水解活性依赖于活性位点锌离子,因此对 MBL 抑制剂的研究涉及锌螯合剂的研究。为了确保此类螯合剂特异性针对 MBL,制备了两种有效的锌结合剂:吡啶二甲酸 (DPA) 和 8-硫代喹啉 (8-TQ) 的一系列头孢菌素前药。尽管 DPA 和 8-TQ 都非常紧密地结合游离锌(K d值在低 nm范围内),但相应的头孢菌素缀合物则不然。NMR 和 LC-MS 研究证实,头孢菌素缀合物可被 MBL 有效水解,释放 DPA 或 8-TQ。值得注意的是,DPA 和 8-TQ 的头孢菌素前药对 NDM、VIM 和 IMP 类 MBL 表现出有效的抑制活性,并与美罗培南对表达 MBL 的肺炎克雷伯菌和大肠杆菌临床分离株表现出有效的协同作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号