当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Bifunctional Sulfide-Catalyzed Highly Enantioselective Bromolactonizations of 4-Pentenoic Acids
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/ajoc.202000644 Ryuichi Nishiyori 1 , Megumi Okada 1 , John R. J. Maynard 1, 2 , Seiji Shirakawa 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/ajoc.202000644 Ryuichi Nishiyori 1 , Megumi Okada 1 , John R. J. Maynard 1, 2 , Seiji Shirakawa 1
Affiliation
|
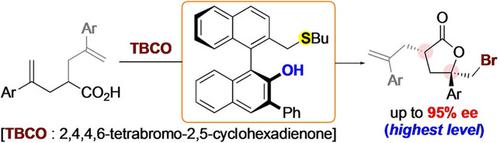
Despite extensive studies into the design of effective chiral catalysts for asymmetric halolactonizations, the development of highly enantioselective catalytic bromolactonization of 4-aryl-4-pentenoic acids, which is one of the benchmark reactions, has not been completely satisfactory. Herein, we report the use of BINOL-derived chiral bifunctional sulfide catalysts to achieve highly enantioselective bromolactonizations of 4-aryl-4-pentenoic acids. The importance of the bifunctional design of chiral sulfide catalysts was clearly demonstrated in the present study. Furthermore, the present catalytic asymmetric reaction system could be applied to highly stereoselective desymmetrizing bromolactonizations.
中文翻译:

手性双功能硫化物催化 4-戊烯酸的高度对映选择性溴内酯化
尽管对用于不对称卤代内酯化的有效手性催化剂的设计进行了广泛的研究,但作为基准反应之一的 4-芳基-4-戊烯酸的高度对映选择性催化溴内酯化的开发并不完全令人满意。在此,我们报告了使用 BINOL 衍生的手性双功能硫化物催化剂来实现 4-芳基-4-戊烯酸的高度对映选择性溴内酯化。本研究清楚地证明了手性硫化物催化剂双功能设计的重要性。此外,本催化不对称反应体系可应用于高度立体选择性的去对称溴内酯化反应。
更新日期:2020-11-24
中文翻译:

手性双功能硫化物催化 4-戊烯酸的高度对映选择性溴内酯化
尽管对用于不对称卤代内酯化的有效手性催化剂的设计进行了广泛的研究,但作为基准反应之一的 4-芳基-4-戊烯酸的高度对映选择性催化溴内酯化的开发并不完全令人满意。在此,我们报告了使用 BINOL 衍生的手性双功能硫化物催化剂来实现 4-芳基-4-戊烯酸的高度对映选择性溴内酯化。本研究清楚地证明了手性硫化物催化剂双功能设计的重要性。此外,本催化不对称反应体系可应用于高度立体选择性的去对称溴内酯化反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号