Redox Biology ( IF 10.7 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.redox.2020.101809 Anke Schmidt 1 , Grit Liebelt 1 , Felix Nießner 1 , Thomas von Woedtke 2 , Sander Bekeschus 1
|
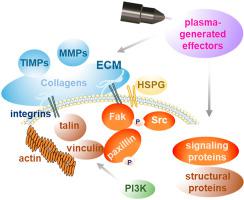
In response to injury, efficient migration of skin cells to rapidly close the wound and restore barrier function requires a range of coordinated processes in cell spreading and migration. Gas plasma technology produces therapeutic reactive species that promote skin regeneration by driving proliferation and angiogenesis. However, the underlying molecular mechanisms regulating gas plasma-aided cell adhesion and matrix remodeling essential for wound closure remain elusive. Here, we combined in vitro analyses in primary dermal fibroblasts isolated from murine skin with in vivo studies in a murine wound model to demonstrate that gas plasma treatment changed phosphorylation of signaling molecules such as focal adhesion kinase and paxillin α in adhesion-associated complexes. In addition to cell spreading and migration, gas plasma exposure affected cell surface adhesion receptors (e.g., integrinα5β1, syndecan 4), structural proteins (e.g., vinculin, talin, actin), and transcription of genes associated with differentiation markers of fibroblasts-to-myofibroblasts and epithelial-to-mesenchymal transition, cellular protrusions, fibronectin fibrillogenesis, matrix metabolism, and matrix metalloproteinase activity. Finally, we documented that gas plasma exposure increased tissue oxygenation and skin perfusion during ROS-driven wound healing. Altogether, these results provide critical insights into the molecular machinery of gas plasma-assisted wound healing mechanisms.
中文翻译:

气体等离子体刺激的伤口愈合伴随着粘着斑、基质重塑和组织氧合的调节
为了响应损伤,皮肤细胞的有效迁移以快速闭合伤口并恢复屏障功能需要一系列细胞扩散和迁移的协调过程。气体等离子体技术产生治疗活性物质,通过驱动增殖和血管生成来促进皮肤再生。然而,调节气体等离子体辅助细胞粘附和伤口闭合所必需的基质重塑的潜在分子机制仍然难以捉摸。在这里,我们将从小鼠皮肤分离的原代真皮成纤维细胞的体外分析与小鼠伤口模型的体内研究相结合,以证明气体等离子体处理改变了粘附相关复合物中信号分子的磷酸化,例如粘着斑激酶和桩蛋白α。除了细胞扩散和迁移之外,气体等离子体暴露还影响细胞表面粘附受体(例如,整联蛋白α5β1、syndecan 4)、结构蛋白(例如,纽蛋白、talin、肌动蛋白)以及与成纤维细胞分化标志物相关的基因转录。肌成纤维细胞和上皮间质转化、细胞突起、纤连蛋白原纤维形成、基质代谢和基质金属蛋白酶活性。最后,我们记录了在 ROS 驱动的伤口愈合过程中,气体等离子体暴露增加了组织氧合和皮肤灌注。总而言之,这些结果为气体等离子体辅助伤口愈合机制的分子机制提供了重要的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号