JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.jchf.2020.10.012 G Michael Felker 1 , John J V McMurray 2 , John G Cleland 3 , Christopher M O'Connor 4 , John R Teerlink 5 , Adriaan A Voors 6 , Jan Belohlavek 7 , Michael Böhm 8 , Maria Borentain 9 , Hector Bueno 10 , Robert T Cole 4 , Mary M DeSouza 9 , Justin A Ezekowitz 11 , Gerasimos Filippatos 12 , Ninian N Lang 2 , Paul D Kessler 9 , Felipe A Martinez 13 , Alex Mebazaa 14 , Marco Metra 15 , Arend Mosterd 16 , Peter S Pang 17 , Piotr Ponikowski 18 , Naoki Sato 19 , Dietmar Seiffert 9 , June Ye 9
|
Objectives
The primary objective was to identify well-tolerated doses of cimlanod in patients with acute heart failure (AHF). Secondary objectives were to identify signals of efficacy, including biomarkers, symptoms, and clinical events.
Background
Nitroxyl (HNO) donors have vasodilator, inotropic and lusitropic effects. Bristol-Myers Squibb-986231 (cimlanod) is an HNO donor being developed for acute heart failure (AHF).
Methods
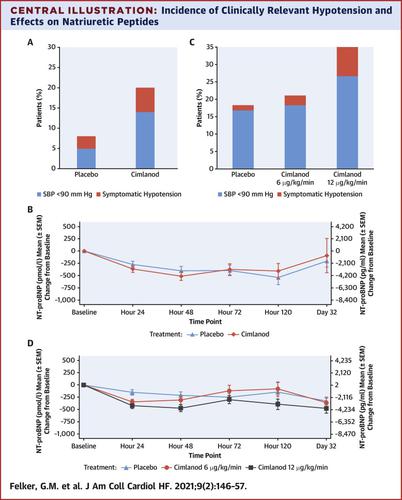
This was a phase IIb, double-blind, randomized, placebo-controlled trial of 48-h treatment with cimlanod compared with placebo in patients with left ventricular ejection fraction ≤40% hospitalized for AHF. In part I, patients were randomized in a 1:1 ratio to escalating doses of cimlanod or matching placebo. In part II, patients were randomized in a 1:1:1 ratio to either of the 2 highest tolerated doses of cimlanod from part I or placebo. The primary endpoint was the rate of clinically relevant hypotension (systolic blood pressure <90 mm Hg or patients became symptomatic).
Results
In part I (n = 100), clinically relevant hypotension was more common with cimlanod than placebo (20% vs. 8%; relative risk [RR]: 2.45; 95% confidence interval [CI]: 0.83 to 14.53). In part II (n = 222), the incidence of clinically relevant hypotension was 18% for placebo, 21% for cimlanod 6 μg/kg/min (RR: 1.15; 95% CI: 0.58 to 2.43), and 35% for cimlanod 12 μg/kg/min (RR: 1.9; 95% CI: 1.04 to 3.59). N-terminal pro–B-type natriuretic peptide and bilirubin decreased during infusion of cimlanod treatment compared with placebo, but these differences did not persist after treatment discontinuation.
Conclusions
Cimlanod at a dose of 6 μg/kg/min was reasonably well-tolerated compared with placebo. Cimlanod reduced markers of congestion, but this did not persist beyond the treatment period. (Evaluate the Safety and Efficacy of 48-Hour Infusions of HNO (Nitroxyl) Donor in Hospitalized Patients With Heart Failure [STANDUP AHF]; NCT03016325)
中文翻译:

新型硝基氧供体在急性心力衰竭中的作用
目标
主要目的是确定急性心力衰竭 (AHF) 患者中耐受良好的西莫诺剂量。次要目标是确定疗效信号,包括生物标志物、症状和临床事件。
背景
硝基酰 (HNO) 供体具有血管扩张剂、肌力作用和向光作用。Bristol-Myers Squibb-986231 (cimlanod) 是一种用于急性心力衰竭 (AHF) 的 HNO 供体。
方法
这是一项 IIb 期、双盲、随机、安慰剂对照试验,在左心室射血分数≤40% 的 AHF 住院患者中进行 48 小时 cimlanod 治疗与安慰剂比较。在第一部分中,患者以 1:1 的比例随机接受递增剂量的 cimlanod 或匹配的安慰剂。在第 II 部分中,患者以 1:1:1 的比例随机分配至第 I 部分中的 2 个最高耐受剂量的西莫诺或安慰剂。主要终点是临床相关低血压的发生率(收缩压 <90 mmHg 或患者出现症状)。
结果
在第 I 部分(n = 100)中,临床相关的低血压在 cimlanod 中比安慰剂更常见(20% 对 8%;相对风险 [RR]:2.45;95% 置信区间 [CI]:0.83 至 14.53)。在第二部分(n = 222)中,安慰剂的临床相关低血压发生率为 18%,cimlanod 6 μg/kg/min 的发生率为 21%(RR:1.15;95% CI:0.58 至 2.43),cimlanod 的发生率为 35% 12 μg/kg/min(RR:1.9;95% CI:1.04 至 3.59)。与安慰剂相比,在输注 cimlanod 治疗期间,N 端前 B 型利钠肽和胆红素降低,但这些差异在治疗中断后不再持续。
结论
与安慰剂相比,6 μg/kg/min 剂量的 Cimlanod 耐受性相当好。Cimlanod 减少了充血标志物,但这在治疗期之后并没有持续。(评估住院心力衰竭患者 48 小时输注 HNO(硝基)供体的安全性和有效性 [STANDUP AHF];NCT03016325)











































 京公网安备 11010802027423号
京公网安备 11010802027423号