Biomaterials ( IF 12.8 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.biomaterials.2020.120574 Man Shu , Junjie Tang , Lili Chen , Qiang Zeng , Chao Li , Shuting Xiao , Zhaozhong Jiang , Jie Liu
|
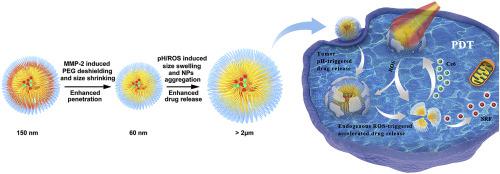
A novel combined chemo/photodynamic therapy has been developed to use pH/ROS/MMP-2 triple-responsive drug nanocarriers for treating solid tumor with an extraordinarily high efficiency. The designed poly(ethylene glycol)-peptide-poly(ω-pentadecalactone-co-N-methyldiethyleneamine-co-3,3′-thiodipropionate) (PEG-M-PPMT) nanoparticles (NPs) encapsulating anticancer drug sorafenib (SRF) and photosensitizer chlorin e6 (Ce6) are stable in serum-containing aqueous media and can effectively accumulate in tumor as a result of the EPR effect after intravenous administration in vivo. In the presence of MMP-2 overexpressed in extracellular tumor matrix, the PEG-M-PPMT NPs can partially shed PEG corona to form smaller particles and penetrate deep into tumor tissue. After uptake by tumor cells, the acidic endosomal pH and high intracellular ROS level would trigger substantial swelling of the NPs to accelerate the drug release for rapid killing of the cancer cells. In the current combined chemo/photodynamic therapy, the intracellular ROS generation in tumor is amplified by photosensitizer Ce6 activated with external laser irradiation. As the result, the highly elevated intracellular ROS concentration can both directly induce apoptosis of ROS-stressed tumor cells and magnify acceleration of the drug release from the ROS-responsive PEG-M-PPMT NPs to gain extraordinary therapeutic efficacy. In particular, after the chemo-photodynamic therapeutic treatment with SRF/Ce6-loaded PEG-M-PPMT nanoparticles, all human lung tumors (A549) xenografted in nude mice shrank substantially with approximately 29% of the tumors being completely eradicated. Additionally, SRF/Ce6-loaded PEG-M-PPMT NPs show negligible in vivo toxicity toward major organs such as heart, liver, spleen, lung and kidney. These results demonstrate great potential of the combined chemo/photodynamic therapy based on the stimuli-responsive PEG-M-PPMT nanoparticles for efficient tumor treatment.
中文翻译:

肿瘤微环境三重响应纳米颗粒可增强肿瘤渗透性和协同化学光动力疗法
已经开发出一种新颖的化学/光动力联合疗法,以使用pH / ROS / MMP-2三重反应药物纳米载体以极高的效率治疗实体瘤。所设计的聚(乙二醇) -肽-聚(ω-pentadecalactone-共- ñ -methyldiethyleneamine-共-3,3'-硫代二丙酸酯)(PEG-M-PPMT)纳米颗粒(NP)包封抗癌药物索拉非尼(SRF)和光敏剂二氢卟酚e6(Ce6)在含血清的水性介质中稳定,并且在体内静脉内给药后由于EPR效应而可以有效地在肿瘤中蓄积。在细胞外肿瘤基质中过表达的MMP-2存在下,PEG-M-PPMT NP可以部分脱落PEG电晕形成较小的颗粒,并深入肿瘤组织。肿瘤细胞摄取后,酸性内体pH和高细胞内ROS水平将触发NP的大量溶胀,从而加速药物释放,从而迅速杀死癌细胞。在当前的化学/光动力联合疗法中,肿瘤内细胞内ROS的产生是通过用外部激光照射激活的光敏剂Ce6来放大的。结果,高度升高的细胞内ROS浓度既可以直接诱导ROS应激的肿瘤细胞凋亡,又可以放大从ROS反应性PEG-M-PPMT NP释放药物的速度,从而获得非凡的治疗效果。尤其是,在用载有SRF / Ce6的PEG-M-PPMT纳米颗粒进行化学光动力治疗后,裸鼠体内异种移植的所有人类肺部肿瘤(A549)明显萎缩,其中约29%的肿瘤被完全根除。此外,加载SRF / Ce6的PEG-M-PPMT NP可忽略不计对主要器官(如心脏,肝脏,脾脏,肺和肾脏)的体内毒性。这些结果证明了基于刺激响应性PEG-M-PPMT纳米粒子的化学/光动力疗法相结合的巨大潜力,可以有效地治疗肿瘤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号