Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.apcatb.2020.119732 Hanxiao Chen , Yin Xu , Kangmeng Zhu , Hui Zhang
|
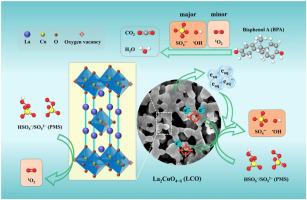
An A2BO4-type oxygen-deficient perovskite (La2CuO4-δ, LCO), which owns a better low-temperature reducibility, more abundant oxygen vacancies (OVs) and surface oxygen species than the benchmark CuO oxide, was first applied in peroxymonosulfate (PMS) activation. At 0.7 g/L of LCO and 2 mM of PMS, more than 96 % of bisphenol A (BPA) was removed within 60 min over a wide pH range of 3.1 ‒ 9.1. The cycle of surface Cu(II)/Cu(I) redox couple and lattice oxygen (O2−)/O2 were responsible for PMS activation. Besides, the OVs with localized electrons may directly activate PMS through single electron transfer pathway to generate surface-bound hydroxyl radical ( OH) and sulfate radical (SO4
OH) and sulfate radical (SO4 −), as well as less singlet oxygen (1O2) for BPA degradation. The evolution of
−), as well as less singlet oxygen (1O2) for BPA degradation. The evolution of  OH, SO4
OH, SO4 − and 1O2 were revealed by EPR tests. Briefly, this study provides the mechanistic understanding of A2BO4-type perovskite in activation of PMS for water treatment.
− and 1O2 were revealed by EPR tests. Briefly, this study provides the mechanistic understanding of A2BO4-type perovskite in activation of PMS for water treatment.
中文翻译:

了解缺氧La 2 CuO4 -δ钙钛矿活化过氧单硫酸盐对双酚A降解的作用:局部电子在氧空位中的作用
首先是A 2 BO 4型缺氧钙钛矿(La 2 CuO4 -δ,LCO),它具有比基准CuO氧化物更好的低温还原性,更丰富的氧空位(OVs)和表面氧种类。用于过一硫酸盐(PMS)活化。在0.7 g / L的LCO和2 mM的PMS下,在3.1 9.1的宽pH范围内,在60分钟内去除了96%以上的双酚A(BPA)。表面Cu(II)/ Cu(I)氧化还原对和晶格氧(O 2-)/ O 2的循环是PMS活化的原因。此外,具有局部电子的OVs可以通过单电子转移途径直接激活PMS,从而产生表面结合的羟基( OH)和硫酸根(SO 4
OH)和硫酸根(SO 4  - ),以及较少的单线态氧(1 Ò 2)为BPA降解。的演变
- ),以及较少的单线态氧(1 Ò 2)为BPA降解。的演变 OH,SO 4
OH,SO 4  -和1 ö 2通过EPR试验揭示。简而言之,该研究提供了在水处理中激活PMS时A 2 BO 4型钙钛矿的机理。
-和1 ö 2通过EPR试验揭示。简而言之,该研究提供了在水处理中激活PMS时A 2 BO 4型钙钛矿的机理。










































 京公网安备 11010802027423号
京公网安备 11010802027423号