Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.apcatb.2020.119735 Piu Chawdhury , Yaolin Wang , Debjyoti Ray , Stéphanie Mathieu , Ni Wang , Jonathan Harding , Feng Bin , Xin Tu , Ch. Subrahmanyam
|
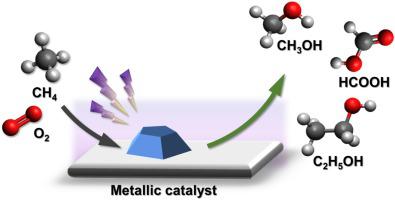
Direct conversion of methane into chemicals and fuels under mild conditions has been considered as a ‘holy grail’ of chemistry and catalysis in the 21st century. Plasma-catalytic partial oxidation of methane (POM) to higher-value liquid fuels and chemicals over supported transition metal catalysts (Ni/γ-Al2O3, Cu/γ-Al2O3 and Fe/γ-Al2O3) has been investigated in a co-axial dielectric barrier discharge (DBD) reactor at room temperature and atmospheric pressure. The selectivity of oxygenates was 58.3% in the plasma POM reaction without a catalyst, while the combination of DBD with the catalysts enhanced the selectivity of oxygenates up to 71.5%. Of the three catalysts, Fe/γ-Al2O3 showed the highest methanol selectivity of 36.0% and a significant methanol yield of 4.7%, while the use of Cu/γ-Al2O3 improved the selectivity of C2 oxygenates to 9.4%, which can be attributed to the presence of more acid sites on the surfaces of the Cu catalyst. The possible reaction pathways in the plasma-catalytic POM reaction have been explored by combined means of plasma electrical and optical diagnostics, analysis of gas and liquid products, as well as comprehensive catalyst characterization. The plausible reaction routes for the production of major oxygenate (methanol) on the Fe/γ-Al2O3 surfaces have been proposed. The surface CHx species are found to be critical for methanol synthesis; they can be formed through the direct adsorption of CHx radicals generated in the plasma gas-phase reactions or through the dissociation of adsorbed CH4 on the catalyst surface.
中文翻译:

一种有望在室温下将甲烷一步转化为含氧化合物的等离子催化方法
在温和的条件下,将甲烷直接转化为化学物质和燃料已被认为是21世纪化学和催化作用的“圣杯”。甲烷(POM)到更高价值的液体燃料和化学品的血浆催化部分氧化过负载的过渡金属催化剂(镍/γ-Al系2 ö 3,铜/γ-Al系2 ö 3和Fe /γ-Al系2 ö 3)已在室温和大气压下的同轴电介质阻挡放电(DBD)反应器中进行了研究。在没有催化剂的情况下,等离子体POM反应中含氧化合物的选择性为58.3%,而DBD与催化剂的组合可将含氧化合物的选择性提高至71.5%。在三种催化剂中,Fe /γ-Al2 Ó 3显示出36.0%的最高的甲醇选择性和4.7%显著甲醇产率,而使用的Cu /γ-Al系2 ö 3提高C的选择性2的含氧化合物至9.4%,这可以归因于存在Cu催化剂表面上更多的酸位。通过等离子电学和光学诊断,气体和液体产物分析以及全面的催化剂表征,探索了等离子体催化POM反应中可能的反应途径。中的Fe /γ-Al系上用于生产主要含氧化合物(甲醇)的似是而非的反应路线2个ö 3表面已经被提出。表面CH x已发现物种对于甲醇合成至关重要;它们可以通过直接吸附在等离子体气相反应中生成的CH x自由基或通过在催化剂表面上解离所吸附的CH 4来形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号