当前位置:
X-MOL 学术
›
Renew. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acetalization of 5-hydroxymethyl furfural into biofuel additive cyclic acetal using protic ionic liquid catalyst- A Thermodynamic and kinetic analysis
Renewable Energy ( IF 9.0 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.renene.2020.11.084 Komal Kumar , Shailesh Pathak , Sreedevi Upadhyayula
Renewable Energy ( IF 9.0 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.renene.2020.11.084 Komal Kumar , Shailesh Pathak , Sreedevi Upadhyayula
|
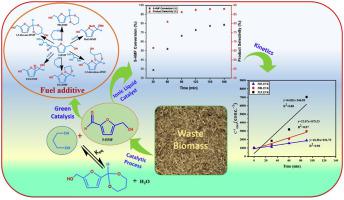
Abstract This work investigates the thermodynamics and kinetics of the synthesis of 5-HMF-cyclic acetal, a fuel additive, from 5-HMF and 1,3-propanediol using lab synthesized, environmentally benign Bronsted acidic ionic liquid (IL) catalyst. Firstly, a thermodynamic study of the acetalization of 5-HMF with different mono and polyalcohols was carried out to evaluate the thermodynamic properties of critical temperature ( T c ), critical volume ( V c ) , critical pressure ( P c ) , Gibbs free energy of formation ( n G f 0 ) , enthalpy of formation ( n f H 0 ) , and heat capacity ( C p ) using group contribution methods. Then, kinetics of 5-HMF acetalization with 1,3-propanediol was investigated in a batch autoclave using Bronsted acidic IL catalyst and effect of reaction parameters temperature, molar ratio of reactants and time were investigated on the conversion of 5-HMF into 5-HMF-cyclic acetal product and optimized for high 5-HMF conversion and cyclic acetal yield. A first-order pseudo-homogeneous kinetic model with a squared regression coefficient R2>0.99 fitted well with experimental data. The activation energy of the acetalization reaction is estimated to be 83 kJ/mol for the 1st order kinetics. Based on the catalyst activity, a plausible reaction mechanism was proposed for the acetalization reaction of 5-HMF and 1,3-propanediol. Finally, this manuscript reports an efficient method for the production of fuel-additive compound and valuable information for predicting the thermodynamic properties for the pure organic components.
中文翻译:

使用质子离子液体催化剂将 5-羟甲基糠醛缩醛化为生物燃料添加剂环状缩醛-热力学和动力学分析
摘要 本工作研究了使用实验室合成的、环境友好的布朗斯台德酸性离子液体 (IL) 催化剂从 5-HMF 和 1,3-丙二醇合成燃料添加剂 5-HMF-环状缩醛的热力学和动力学。首先,对5-HMF与不同的一元醇和多元醇的缩醛化进行了热力学研究,评估了临界温度( T c )、临界体积( V c )、临界压力( P c )、吉布斯自由能的热力学性质。使用群贡献方法计算形成 (n G f 0 )、形成焓 (nf H 0 ) 和热容量 (C p )。然后,使用布朗斯台德酸性 IL 催化剂在间歇高压釜中研究 5-HMF 与 1,3-丙二醇缩醛化的动力学以及反应参数温度的影响,研究了反应物的摩尔比和时间对 5-HMF 向 5-HMF-环状缩醛产物的转化,并针对高 5-HMF 转化率和环状缩醛产率进行了优化。具有平方回归系数R2>0.99的一阶伪均相动力学模型与实验数据拟合良好。对于一级动力学,缩醛化反应的活化能估计为 83 kJ/mol。基于催化剂活性,提出了 5-HMF 和 1,3-丙二醇的缩醛化反应的合理反应机理。最后,这份手稿报告了一种生产燃料添加剂化合物的有效方法和用于预测纯有机组分热力学性质的宝贵信息。
更新日期:2021-04-01
中文翻译:

使用质子离子液体催化剂将 5-羟甲基糠醛缩醛化为生物燃料添加剂环状缩醛-热力学和动力学分析
摘要 本工作研究了使用实验室合成的、环境友好的布朗斯台德酸性离子液体 (IL) 催化剂从 5-HMF 和 1,3-丙二醇合成燃料添加剂 5-HMF-环状缩醛的热力学和动力学。首先,对5-HMF与不同的一元醇和多元醇的缩醛化进行了热力学研究,评估了临界温度( T c )、临界体积( V c )、临界压力( P c )、吉布斯自由能的热力学性质。使用群贡献方法计算形成 (n G f 0 )、形成焓 (nf H 0 ) 和热容量 (C p )。然后,使用布朗斯台德酸性 IL 催化剂在间歇高压釜中研究 5-HMF 与 1,3-丙二醇缩醛化的动力学以及反应参数温度的影响,研究了反应物的摩尔比和时间对 5-HMF 向 5-HMF-环状缩醛产物的转化,并针对高 5-HMF 转化率和环状缩醛产率进行了优化。具有平方回归系数R2>0.99的一阶伪均相动力学模型与实验数据拟合良好。对于一级动力学,缩醛化反应的活化能估计为 83 kJ/mol。基于催化剂活性,提出了 5-HMF 和 1,3-丙二醇的缩醛化反应的合理反应机理。最后,这份手稿报告了一种生产燃料添加剂化合物的有效方法和用于预测纯有机组分热力学性质的宝贵信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号