Redox Biology ( IF 10.7 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.redox.2020.101808 Csaba Hegedűs 1 , Tamás Juhász 2 , Eszter Fidrus 1 , Eszter Anna Janka 3 , Gábor Juhász 4 , Gábor Boros 5 , György Paragh 6 , Karen Uray 7 , Gabriella Emri 3 , Éva Remenyik 3 , Péter Bai 8
|
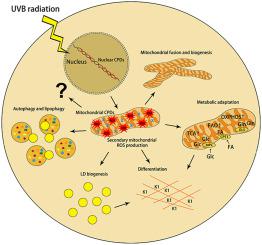
Ultraviolet B radiation (UVB) is an environmental complete carcinogen, which induces and promotes keratinocyte carcinomas, the most common human malignancies. UVB induces the formation of cyclobutane pyrimidine dimers (CPDs). Repairing CPDs through nucleotide excision repair is slow and error-prone in placental mammals. In addition to the mutagenic and malignancy-inducing effects, UVB also elicits poorly understood complex metabolic changes in keratinocytes, possibly through CPDs. To determine the effects of CPDs, CPD-photolyase was overexpressed in keratinocytes using an N1-methyl pseudouridine-containing in vitro-transcribed mRNA. CPD-photolyase, which is normally not present in placental mammals, can efficiently and rapidly repair CPDs to block signaling pathways elicited by CPDs. Keratinocytes surviving UVB irradiation turn hypermetabolic. We show that CPD-evoked mitochondrial reactive oxygen species production, followed by the activation of several energy sensor enzymes, including sirtuins, AMPK, mTORC1, mTORC2, p53, and ATM, is responsible for the compensatory metabolic adaptations in keratinocytes surviving UVB irradiation. Compensatory metabolic changes consist of enhanced glycolytic flux, Szent-Györgyi-Krebs cycle, and terminal oxidation. Furthermore, mitochondrial fusion, mitochondrial biogenesis, and lipophagy characterize compensatory hypermetabolism in UVB-exposed keratinocytes. These properties not only support the survival of keratinocytes, but also contribute to UVB-induced differentiation of keratinocytes. Our results indicate that CPD-dependent signaling acutely maintains skin integrity by supporting cellular energy metabolism.
中文翻译:

UVB暴露引起的环丁烷嘧啶二聚体通过线粒体氧化应激诱导角质形成细胞过度代谢
紫外线B辐射(UVB)是一种环境完全的致癌物,可诱发和促进角化细胞癌,这是人类最常见的恶性肿瘤。UVB诱导形成环丁烷嘧啶二聚体(CPD)。在胎盘哺乳动物中,通过核苷酸切除修复来修复CPD是缓慢且容易出错的。除了诱变和恶性诱导作用外,UVB还可能通过CPD引起人们对角质形成细胞复杂的代谢变化知之甚少。为了确定CPD的作用,体外使用含N1-甲基假尿苷的CPD-光解酶在角质形成细胞中过表达转录的mRNA。通常在胎盘哺乳动物中不存在的CPD-光解酶可以有效,快速地修复CPD,以阻断CPD引发的信号传导途径。幸存于UVB照射下的角质形成细胞代谢过度。我们表明CPD诱发线粒体活性氧的产生,然后激活了几种能量传感器酶,包括sirtuins,AMPK,mTORC1,mTORC2,p53和ATM,负责角化细胞在UVB辐射下的代偿性适应性适应。代偿性代谢变化包括增强的糖酵解通量,Szent-Györgyi-Krebs循环和末端氧化。此外,线粒体融合,线粒体的生物发生和脂肪吞噬的特点是暴露于UVB的角质形成细胞的代偿性高代谢。这些特性不仅支持角质形成细胞的生存,但也有助于UVB诱导的角质形成细胞分化。我们的结果表明,依赖CPD的信号传导通过支持细胞能量代谢而急性维持皮肤完整性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号