Neuron ( IF 14.7 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.neuron.2020.11.002 Minghui Wang 1 , Aiqun Li 2 , Michiko Sekiya 3 , Noam D Beckmann 4 , Xiuming Quan 5 , Nadine Schrode 6 , Michael B Fernando 6 , Alex Yu 6 , Li Zhu 7 , Jiqing Cao 7 , Liwei Lyu 6 , Emrin Horgusluoglu 1 , Qian Wang 1 , Lei Guo 1 , Yuan-Shuo Wang 1 , Ryan Neff 1 , Won-Min Song 1 , Erming Wang 1 , Qi Shen 1 , Xianxiao Zhou 1 , Chen Ming 1 , Seok-Man Ho 6 , Sezen Vatansever 1 , H Ümit Kaniskan 8 , Jian Jin 9 , Ming-Ming Zhou 10 , Kanae Ando 11 , Lap Ho 1 , Paul A Slesinger 12 , Zhenyu Yue 13 , Jun Zhu 14 , Pavel Katsel 15 , Sam Gandy 16 , Michelle E Ehrlich 17 , Valentina Fossati 18 , Scott Noggle 18 , Dongming Cai 7 , Vahram Haroutunian 19 , Koichi M Iijima 3 , Eric Schadt 4 , Kristen J Brennand 6 , Bin Zhang 1
|
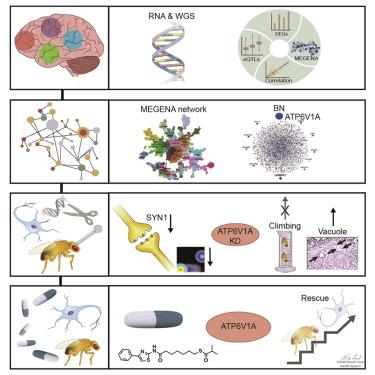
To identify the molecular mechanisms and novel therapeutic targets of late-onset Alzheimer’s Disease (LOAD), we performed an integrative network analysis of multi-omics profiling of four cortical areas across 364 donors with varying cognitive and neuropathological phenotypes. Our analyses revealed thousands of molecular changes and uncovered neuronal gene subnetworks as the most dysregulated in LOAD. ATP6V1A was identified as a key regulator of a top-ranked neuronal subnetwork, and its role in disease-related processes was evaluated through CRISPR-based manipulation in human induced pluripotent stem cell-derived neurons and RNAi-based knockdown in Drosophila models. Neuronal impairment and neurodegeneration caused by ATP6V1A deficit were improved by a repositioned compound, NCH-51. This study provides not only a global landscape but also detailed signaling circuits of complex molecular interactions in key brain regions affected by LOAD, and the resulting network models will serve as a blueprint for developing next-generation therapeutic agents against LOAD.
中文翻译:

多组学数据的变革性网络建模揭示了阿尔茨海默病的详细电路、关键调节器和潜在治疗方法
为了确定迟发性阿尔茨海默病 (LOAD) 的分子机制和新的治疗靶点,我们对 364 名具有不同认知和神经病理学表型的供体的四个皮质区域进行了多组学分析的综合网络分析。我们的分析揭示了数以千计的分子变化,并发现神经元基因子网络是 LOAD 中最失调的。ATP6V1A被确定为排名靠前的神经元子网络的关键调节因子,其在疾病相关过程中的作用通过基于 CRISPR 的人类诱导多能干细胞衍生神经元操作和果蝇模型中基于 RNAi 的敲低进行评估。ATP6V1A引起的神经元损伤和神经变性重新定位的化合物 NCH-51 改善了缺陷。这项研究不仅提供了全球概况,还提供了受 LOAD 影响的关键大脑区域复杂分子相互作用的详细信号通路,由此产生的网络模型将作为开发下一代 LOAD 治疗药物的蓝图。











































 京公网安备 11010802027423号
京公网安备 11010802027423号