Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.jpha.2020.11.006 Xinling Cui 1 , Wei Mi 2 , Zhishang Hu 3 , Xiaoyu Li 1 , Bo Meng 1 , Xinyuan Zhao 1 , Xiaohong Qian 1 , Tao Zhu 4 , Wantao Ying 1
|
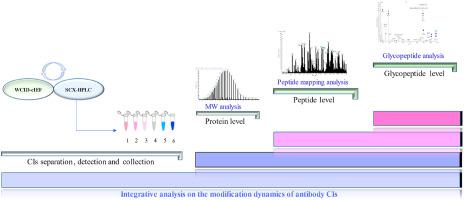
Posttranslational modifications of antibody products affect their stability, charge distribution, and drug activity and are thus a critical quality attribute. The comprehensive mapping of antibody modifications and different charge isomers (CIs) is of utmost importance, but is challenging. We intended to quantitatively characterize the posttranslational modification status of CIs of antibody drugs and explore the impact of posttranslational modifications on charge heterogeneity. The CIs of antibodies were fractionated by strong cation exchange chromatography and verified by capillary isoelectric focusing-whole column imaging detection, followed by stepwise structural characterization at three levels. First, the differences between CIs were explored at the intact protein level using a top-down mass spectrometry approach; this showed differences in glycoforms and deamidation status. Second, at the peptide level, common modifications of oxidation, deamidation, and glycosylation were identified. Peptide mapping showed nonuniform deamidation and glycoform distribution among CIs. In total, 10 N-glycoforms were detected by peptide mapping. Finally, an in-depth analysis of glycan variants of CIs was performed through the detection of enriched glycopeptides. Qualitative and quantitative analyses demonstrated the dynamics of 24 N-glycoforms. The results revealed that sialic acid modification is a critical factor accounting for charge heterogeneity, which is otherwise missed in peptide mapping and intact molecular weight analyses. This study demonstrated the importance of the comprehensive analyses of antibody CIs and provides a reference method for the quality control of biopharmaceutical analysis.
中文翻译:

IgG抗体电荷异构体修饰的全局表征
抗体产品的翻译后修饰会影响其稳定性、电荷分布和药物活性,因此是关键的质量属性。抗体修饰和不同电荷异构体 (CI) 的全面映射至关重要,但具有挑战性。我们打算定量表征抗体药物 CI 的翻译后修饰状态,并探讨翻译后修饰对电荷异质性的影响。通过强阳离子交换色谱分离抗体的CIs,并通过毛细管等电聚焦-全柱成像检测验证,然后在三个水平上进行逐步结构表征。首先,使用自上而下的质谱方法在完整蛋白质水平上探索 CI 之间的差异;这表明糖型和脱酰胺状态存在差异。其次,在肽水平上,确定了氧化、脱酰胺和糖基化的常见修饰。肽图显示 CI 之间的脱酰胺和糖型分布不均匀。通过肽图分析总共检测到 10 种 N-糖型。最后,通过检测富集的糖肽对 CI 的聚糖变体进行了深入分析。定性和定量分析证明了 24 N-糖型的动力学。结果表明,唾液酸修饰是导致电荷异质性的关键因素,否则在肽图分析和完整分子量分析中会忽略这一点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号