Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.chembiol.2020.11.005 Gabriëlle B A van Tilburg 1 , Andrea G Murachelli 2 , Alexander Fish 2 , Gerbrand J van der Heden van Noort 1 , Huib Ovaa 1 , Titia K Sixma 2
|
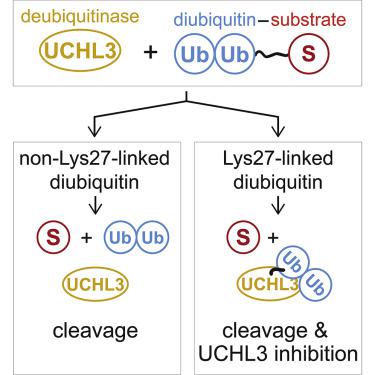
Functional analysis of lysine 27-linked ubiquitin chains (K27Ub) is difficult due to the inability to make them through enzymatic methods and due to a lack of model tools and substrates. Here we generate a series of ubiquitin (Ub) tools to study how the deubiquitinase UCHL3 responds to K27Ub chains in comparison to lysine 63-linked chains and mono-Ub. From a crystal structure of a complex between UCHL3 and synthetic K27Ub2, we unexpectedly discover that free K27Ub2 and K27Ub2-conjugated substrates are natural inhibitors of UCHL3. Using our Ub tools to profile UCHL3's activity, we generate a quantitative kinetic model of the inhibitory mechanism and we find that K27Ub2 can inhibit UCHL3 covalently, by binding to its catalytic cysteine, and allosterically, by locking its catalytic loop tightly in place. Based on this inhibition mechanism, we propose that UCHL3 and K27Ub chains likely sense and regulate each other in cells.
中文翻译:

K27-Linked Diubiquitin 通过不寻常的动力学陷阱抑制 UCHL3
赖氨酸 27 连接的泛素链 ( K27 Ub) 的功能分析很困难,因为无法通过酶促方法制造它们,并且缺乏模型工具和底物。在这里,我们生成了一系列泛素 (Ub) 工具来研究去泛素化酶 UCHL3与赖氨酸 63 连接链和单 Ub 相比如何响应K27 Ub 链。从UCHL3和合成K27 Ub 2复合物的晶体结构中,我们意外地发现游离的K27 Ub 2和K27 Ub 2结合底物是 UCHL3 的天然抑制剂。使用我们的 Ub 工具来分析 UCHL3 的活性,我们生成了抑制机制的定量动力学模型,我们发现K27 Ub 2可以共价抑制 UCHL3,通过与其催化半胱氨酸结合,并通过变构方式将其催化环紧紧锁定到位。基于这种抑制机制,我们提出 UCHL3 和K27 Ub 链可能在细胞中相互感知和调节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号