Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.bmc.2020.115887 Weiguo Xiang 1 , Tasdique M Quadery 1 , Ernest Hamel 2 , Lerin R Luckett-Chastain 3 , Michael A Ihnat 3 , Susan L Mooberry 4 , Aleem Gangjee 1
|
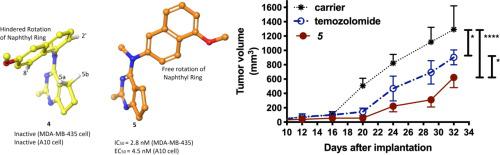
A series of methoxy naphthyl substituted cyclopenta[d]pyrimidine compounds, 4–10, were designed and synthesized to study the influence of the 3-D conformation on microtubule depolymerizing and antiproliferative activities. NOESY studies with the N,2-dimethyl-N-(6ʹ-methoxynaphthyl-1ʹ-amino)-cyclopenta[d]pyrimidin-4-amine (4) showed hindered rotation of the naphthyl ring around the cyclopenta[d]pyrimidine scaffold. In contrast, NOESY studies with N,2-dimethyl-N-(5ʹ-methoxynaphthyl-2ʹ-amino)-cyclopenta[d]pyrimidin-4-amine (5) showed free rotation of the naphthyl ring around the cyclopenta[d]pyrimidine scaffold. The rotational flexibility and conformational dissimilarity between 4 and 5 led to a significant difference in biological activities. Compound 4 is inactive while 5 is the most potent in this series with potent microtubule depolymerizing effects and low nanomolar IC50 values in vitro against a variety of cancer cell lines. The ability of 5 to inhibit tumor growth in vivo was investigated in a U251 glioma xenograft model. The results show that 5 had better antitumor effects than the positive control temozolomide and have identified 5 as a potential preclinical candidate for further studies. The influence of conformation on the microtubule depolymerizing and antitumor activity forms the basis for the development of conformation-activity relationships for the cyclopenta[d]pyrimidine class of microtubule targeting agents.
中文翻译:

N-萘基-环戊[d]嘧啶的 3-D 构象影响其作为微管靶向剂的效力及其抗肿瘤活性
设计并合成了一系列甲氧基萘基取代的环戊[ d ]嘧啶化合物4-10 ,以研究3-D构象对微管解聚和抗增殖活性的影响。 NOESY 对N ,2-二甲基-N- (6ʹ-甲氧基萘基-1ʹ-氨基)-环戊[ d ]嘧啶-4-胺 ( 4 ) 的研究表明萘环围绕环戊[ d ]嘧啶支架的旋转受到阻碍。相比之下,NOESY 对N ,2-二甲基-N- (5ʹ-甲氧基萘基-2ʹ-氨基)-环戊[ d ]嘧啶-4-胺 ( 5 ) 的研究表明,萘环围绕环戊[ d ]嘧啶自由旋转脚手架。 4和5之间的旋转灵活性和构象差异导致生物活性的显着差异。化合物4没有活性,而5是该系列中最有效的化合物,具有有效的微管解聚作用,并且在体外针对多种癌细胞系具有低纳摩尔 IC 50值。在 U251 神经胶质瘤异种移植模型中研究了5抑制体内肿瘤生长的能力。结果表明, 5比阳性对照替莫唑胺具有更好的抗肿瘤作用,并确定5作为进一步研究的潜在临床前候选药物。 构象对微管解聚和抗肿瘤活性的影响构成了环戊[ d ]嘧啶类微管靶向剂构象-活性关系发展的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号