Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.bbapap.2020.140575 Natalia Sasoni , Matías D. Hartman , Sergio A. Guerrero , Alberto A. Iglesias , Diego G. Arias
|
Background
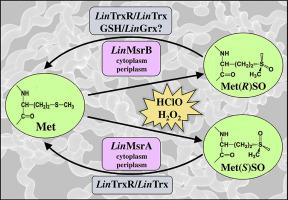
Methionine (Met) oxidation leads to a racemic mixture of R and S forms of methionine sulfoxide (MetSO). Methionine sulfoxide reductases (Msr) are enzymes that can reduce specifically each isomer of MetSO, both free and protein-bound. The Met oxidation could change the structure and function of many proteins, not only of those redox-related but also of others involved in different metabolic pathways. Until now, there is no information about the presence or function of Msrs enzymes in Leptospira interrogans.
Methods
We identified genes coding for putative MsrAs (A1 and A2) and MsrB in L. interrogans serovar Copenhageni strain Fiocruz L1-130 genome project. From these, we obtained the recombinant proteins and performed their functional characterization.
Results
The recombinant L. interrogans MsrB catalyzed the reduction of Met(R)SO using glutaredoxin and thioredoxin as reducing substrates and behaves like a 1-Cys Msr (without resolutive Cys residue). It was able to partially revert the in vitro HClO-dependent inactivation of L. interrogans catalase. Both recombinant MsrAs reduced Met(S)SO, being the recycle mediated by the thioredoxin system. LinMsrAs were more efficient than LinMsrB for free and protein-bound MetSO reduction. Besides, LinMsrAs are enzymes involving a Cys triad in their catalytic mechanism. LinMsrs showed a dual localization, both in cytoplasm and periplasm.
Conclusions and General significance
This article brings new knowledge about redox metabolism in L. interrogans. Our results support the occurrence of a metabolic pathway involved in the critical function of repairing oxidized macromolecules in this pathogen.
中文翻译:

钩端螺旋体甲硫氨酸亚砜还原酶的功能表征
背景
蛋氨酸(Met)氧化会导致R和S形式的蛋氨酸亚砜(MetSO)外消旋混合物。蛋氨酸亚砜还原酶(Msr)是可以特异性还原MetSO的每个异构体的酶,无论是游离的还是蛋白结合的。Met氧化可以改变许多蛋白质的结构和功能,不仅改变那些与氧化还原有关的蛋白质,而且改变其他参与不同代谢途径的蛋白质。到目前为止,还没有关于问号钩端螺旋体中Msrs酶的存在或功能的信息。
方法
我们在L. interrogans serovar Copenhageni菌株Fiocruz L1-130基因组计划中确定了编码假定的MsrAs(A1和A2)和MsrB的基因。从这些中,我们获得了重组蛋白并进行了功能表征。
结果
重组的问号乳酸杆菌MsrB使用戊二醛和硫氧还蛋白作为还原底物催化Met(R)SO的还原,其行为类似于1-Cys Msr(无可分辨的Cys残基)。它能够部分逆转询问乳杆菌过氧化氢酶的体外HClO依赖性失活。两种重组MsrAs都还原了Met(S)SO,这是由硫氧还蛋白系统介导的循环。Lin MsrAs在游离和结合蛋白的MetSO还原方面比Lin MsrB更有效。此外,Lin MsrAs是在其催化机理中涉及Cys三联体的酶。林Msrs在细胞质和周质中均显示出双重定位。
结论与一般意义
本文带来了关于询问乳杆菌氧化还原代谢的新知识。我们的研究结果支持了涉及这种病原体修复氧化大分子关键功能的代谢途径的发生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号