当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ClpP1P2 peptidase activity promotes biofilm formation in Pseudomonas aeruginosa
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-24 , DOI: 10.1111/mmi.14649 Gina D Mawla 1 , Branwen M Hall 1 , Gerardo Cárcamo-Oyarce 2 , Robert A Grant 1 , Jia Jia Zhang 1 , Julia R Kardon 3 , Katharina Ribbeck 2 , Robert T Sauer 1 , Tania A Baker 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-11-24 , DOI: 10.1111/mmi.14649 Gina D Mawla 1 , Branwen M Hall 1 , Gerardo Cárcamo-Oyarce 2 , Robert A Grant 1 , Jia Jia Zhang 1 , Julia R Kardon 3 , Katharina Ribbeck 2 , Robert T Sauer 1 , Tania A Baker 1
Affiliation
|
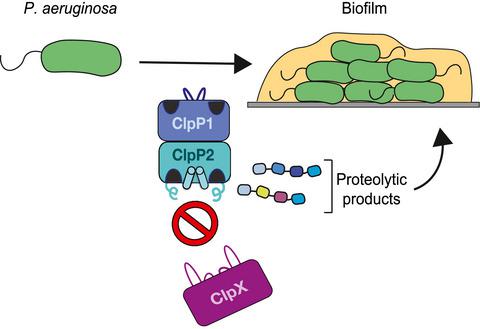
Caseinolytic proteases (Clp) are central to bacterial proteolysis and control cellular physiology and stress responses. They are composed of a double-ring compartmentalized peptidase (ClpP) and a AAA+ unfoldase (ClpX or ClpA/ClpC). Unlike many bacteria, the opportunistic pathogen Pseudomonas aeruginosa contains two ClpP homologs: ClpP1 and ClpP2. The specific functions of these homologs, however, are largely elusive. Here, we report that the active form of PaClpP2 is a part of a heteromeric PaClpP17P27 tetradecamer that is required for proper biofilm development. PaClpP114 and PaClpP17P27 complexes exhibit distinct peptide cleavage specificities and interact differentially with P. aeruginosa ClpX and ClpA. Crystal structures reveal that PaClpP2 has non-canonical features in its N- and C-terminal regions that explain its poor interaction with unfoldases. However, experiments in vivo indicate that the PaClpP2 peptidase active site uniquely contributes to biofilm development. These data strongly suggest that the specificity of different classes of ClpP peptidase subunits contributes to the biological outcome of proteolysis. This specialized role of PaClpP2 highlights it as an attractive target for developing antimicrobial agents that interfere specifically with late-stage P. aeruginosa development.
中文翻译:

ClpP1P2 肽酶活性促进铜绿假单胞菌生物膜形成
酪蛋白溶解蛋白酶 (Clp) 是细菌蛋白水解的核心,并控制细胞生理和应激反应。它们由双环区室化肽酶 (ClpP) 和 AAA+ 解折叠酶(ClpX 或 ClpA/ClpC)组成。与许多细菌不同,机会致病菌铜绿假单胞菌含有两种 ClpP 同源物:ClpP1 和 ClpP2。然而,这些同系物的具体功能在很大程度上是难以捉摸的。在这里,我们报道 PaClpP2 的活性形式是异聚 PaClpP1 7 P2 7四聚体的一部分,这是正确生物膜发育所必需的。 PaClpP1 14和 PaClpP1 7 P2 7复合物表现出不同的肽切割特异性,并与铜绿假单胞菌ClpX 和 ClpA 发生不同的相互作用。晶体结构表明 PaClpP2 在其 N 端和 C 端区域具有非典型特征,这解释了它与去折叠酶的相互作用较差。然而,体内实验表明 PaClpP2 肽酶活性位点对生物膜的形成有独特的贡献。这些数据强烈表明不同类别 ClpP 肽酶亚基的特异性有助于蛋白水解的生物学结果。 PaClpP2 的这种特殊作用凸显了它作为开发特异性干扰晚期铜绿假单胞菌发育的抗菌药物的一个有吸引力的目标。
更新日期:2020-11-24
中文翻译:

ClpP1P2 肽酶活性促进铜绿假单胞菌生物膜形成
酪蛋白溶解蛋白酶 (Clp) 是细菌蛋白水解的核心,并控制细胞生理和应激反应。它们由双环区室化肽酶 (ClpP) 和 AAA+ 解折叠酶(ClpX 或 ClpA/ClpC)组成。与许多细菌不同,机会致病菌铜绿假单胞菌含有两种 ClpP 同源物:ClpP1 和 ClpP2。然而,这些同系物的具体功能在很大程度上是难以捉摸的。在这里,我们报道 PaClpP2 的活性形式是异聚 PaClpP1 7 P2 7四聚体的一部分,这是正确生物膜发育所必需的。 PaClpP1 14和 PaClpP1 7 P2 7复合物表现出不同的肽切割特异性,并与铜绿假单胞菌ClpX 和 ClpA 发生不同的相互作用。晶体结构表明 PaClpP2 在其 N 端和 C 端区域具有非典型特征,这解释了它与去折叠酶的相互作用较差。然而,体内实验表明 PaClpP2 肽酶活性位点对生物膜的形成有独特的贡献。这些数据强烈表明不同类别 ClpP 肽酶亚基的特异性有助于蛋白水解的生物学结果。 PaClpP2 的这种特殊作用凸显了它作为开发特异性干扰晚期铜绿假单胞菌发育的抗菌药物的一个有吸引力的目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号