Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of solvent polarity on excited state behaviors for the two intramolecular proton-transfer-site 4,4’-(hydrazine-1,2-diylidene-bis(methanylylidene))-bis(3-hydroxybenzoic acid) compound
Journal of Luminescence ( IF 3.3 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.jlumin.2020.117800 Jinfeng Zhao , Bing Jin
Journal of Luminescence ( IF 3.3 ) Pub Date : 2021-04-01 , DOI: 10.1016/j.jlumin.2020.117800 Jinfeng Zhao , Bing Jin
|
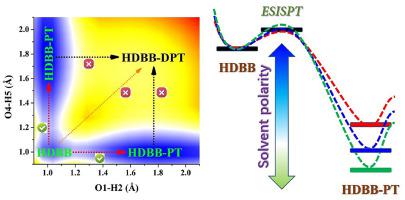
Abstract Excited state proton transfer (ESPT) behavior of organic fluorophores has been drawing continuously considerable interests, since it owns enormous potential in wide of application fields. Given the paramount importance of excited state relaxations, in this work, we focus on deciphering excited state behaviors for the novel two proton-transfer-site 4,4’-(hydrazine-1,2-diylidene-bis(methanylylidene))-bis(3-hydroxybenzoic acid) (HDBB) system. In aprotic solvents with different polarities, we confirm the dual hydrogen bonds of HDBB should be enhanced in S1 state. It is a remarkable fact that nonpolar solvents play a more vital role in enhancing dual hydrogen bonds in S1 state. Probing into photo-induced excitation, we verify charge reorganization around proton acceptor and donor triggers ESPT tendency. Comparing energy gaps of frontier molecular orbitals (MOs), we further predict the ESPT process of HDBB could be facilitated by nonpolar solvents. To clarify the specific excited state behaviors, potential energy surfaces (PESs) in different solvents are constructed along with dual hydrogen bonds to present the overall ESPT processes. Searching transition state (TS) along reaction path, we confirm the excited state intramolecular single proton transfer (ESISPT) mechanism for HDBB. Further, we successfully present the excited state regulation mechanism via solvent polarity, namely, nonpolar solvents contribute to ESISPT reaction for HDBB compound.
中文翻译:

溶剂极性对两个分子内质子转移位点 4,4'-(hydrazine-1,2-diylidene-bis(methanylylidene))-bis(3-hydroxybenzoic acid) 化合物激发态行为的影响
摘要 有机荧光团的激发态质子转移(ESPT)行为一直受到广泛关注,因为它在广泛的应用领域拥有巨大的潜力。鉴于激发态弛豫的重要性,在这项工作中,我们专注于破译新型两个质子转移位点 4,4'-(hydrazine-1,2-diylidene-bis(methanylylidene))-bis 的激发态行为(3-羟基苯甲酸)(HDBB)体系。在具有不同极性的非质子溶剂中,我们确认 HDBB 的双氢键在 S1 状态下应增强。一个值得注意的事实是,非极性溶剂在增强 S1 状态的双氢键方面起着更重要的作用。探讨光致激发,我们验证了质子受体和供体周围的电荷重组触发 ESPT 趋势。比较前沿分子轨道(MO)的能隙,我们进一步预测非极性溶剂可以促进 HDBB 的 ESPT 过程。为了阐明特定的激发态行为,不同溶剂中的势能面 (PES) 与双氢键一起构建,以呈现整个 ESPT 过程。沿着反应路径搜索过渡态 (TS),我们确认了 HDBB 的激发态分子内单质子转移 (ESISPT) 机制。此外,我们成功地通过溶剂极性提出了激发态调节机制,即非极性溶剂有助于 HDBB 化合物的 ESISPT 反应。不同溶剂中的势能面 (PES) 与双氢键一起构建,以呈现整体 ESPT 过程。沿着反应路径搜索过渡态 (TS),我们确认了 HDBB 的激发态分子内单质子转移 (ESISPT) 机制。此外,我们成功地通过溶剂极性提出了激发态调节机制,即非极性溶剂有助于 HDBB 化合物的 ESISPT 反应。不同溶剂中的势能面 (PES) 与双氢键一起构建,以呈现整体 ESPT 过程。沿着反应路径搜索过渡态 (TS),我们确认了 HDBB 的激发态分子内单质子转移 (ESISPT) 机制。此外,我们成功地通过溶剂极性提出了激发态调节机制,即非极性溶剂有助于 HDBB 化合物的 ESISPT 反应。
更新日期:2021-04-01
中文翻译:

溶剂极性对两个分子内质子转移位点 4,4'-(hydrazine-1,2-diylidene-bis(methanylylidene))-bis(3-hydroxybenzoic acid) 化合物激发态行为的影响
摘要 有机荧光团的激发态质子转移(ESPT)行为一直受到广泛关注,因为它在广泛的应用领域拥有巨大的潜力。鉴于激发态弛豫的重要性,在这项工作中,我们专注于破译新型两个质子转移位点 4,4'-(hydrazine-1,2-diylidene-bis(methanylylidene))-bis 的激发态行为(3-羟基苯甲酸)(HDBB)体系。在具有不同极性的非质子溶剂中,我们确认 HDBB 的双氢键在 S1 状态下应增强。一个值得注意的事实是,非极性溶剂在增强 S1 状态的双氢键方面起着更重要的作用。探讨光致激发,我们验证了质子受体和供体周围的电荷重组触发 ESPT 趋势。比较前沿分子轨道(MO)的能隙,我们进一步预测非极性溶剂可以促进 HDBB 的 ESPT 过程。为了阐明特定的激发态行为,不同溶剂中的势能面 (PES) 与双氢键一起构建,以呈现整个 ESPT 过程。沿着反应路径搜索过渡态 (TS),我们确认了 HDBB 的激发态分子内单质子转移 (ESISPT) 机制。此外,我们成功地通过溶剂极性提出了激发态调节机制,即非极性溶剂有助于 HDBB 化合物的 ESISPT 反应。不同溶剂中的势能面 (PES) 与双氢键一起构建,以呈现整体 ESPT 过程。沿着反应路径搜索过渡态 (TS),我们确认了 HDBB 的激发态分子内单质子转移 (ESISPT) 机制。此外,我们成功地通过溶剂极性提出了激发态调节机制,即非极性溶剂有助于 HDBB 化合物的 ESISPT 反应。不同溶剂中的势能面 (PES) 与双氢键一起构建,以呈现整体 ESPT 过程。沿着反应路径搜索过渡态 (TS),我们确认了 HDBB 的激发态分子内单质子转移 (ESISPT) 机制。此外,我们成功地通过溶剂极性提出了激发态调节机制,即非极性溶剂有助于 HDBB 化合物的 ESISPT 反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号