当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Pyridone-stabilized iridium silylene/silyl complexes: structure and QTAIM analysis
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-11-19 , DOI: 10.1039/d0dt03326j Jefferson Guzmán 1, 2, 3, 4, 5 , Ana M. Bernal 1, 2, 3, 4, 5 , Pilar García-Orduña 1, 2, 3, 4, 5 , Fernando J. Lahoz 1, 2, 3, 4, 5 , Víctor Polo 3, 4, 5, 6, 7 , Francisco J. Fernández-Alvarez 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2020-11-19 , DOI: 10.1039/d0dt03326j Jefferson Guzmán 1, 2, 3, 4, 5 , Ana M. Bernal 1, 2, 3, 4, 5 , Pilar García-Orduña 1, 2, 3, 4, 5 , Fernando J. Lahoz 1, 2, 3, 4, 5 , Víctor Polo 3, 4, 5, 6, 7 , Francisco J. Fernández-Alvarez 1, 2, 3, 4, 5
Affiliation
|
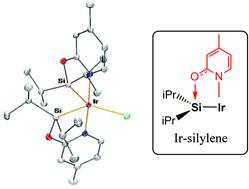
Iridium(III) complexes of the general formula [Ir(X)(κ2-NSiiPr2)2] (NSiiPr2 = (4-methyl-pyridine-2-yloxy)diisopropylsilyl; X = Cl, 3; CF3SO3, 5; CF3CO2, 6) have been prepared and fully characterized, including X-ray diffraction studies and theoretical calculations. The presence of isopropyl substituents at the silicon atom favours the monomeric structure found in complexes 3 and 5. The short Ir–Si bond distances (2.25–2.28 Å) indicate some degree of base-stabilized silylene character of the Ir–Si bond in 3, 5 and 6 assisted by the 2-pyridone moiety. However, the shortening of these Ir–Si bonds might be a consequence of the constrained 2-pyridone geometry, and consequently the silyl character of these bonds can not be excluded. A DFT theoretical study on the nature of the Ir–Si bonds has been performed for complex 3 as well as for four other iridium complexes finding representative examples of different bonding situations between Ir and Si atoms: silylene, base-assisted silylene (both with an anionic base and with a neutral base), and silyl bonds, using the topological properties of the electron charge density. The results of these studies show that the Ir–Si bonds in Ir–NSiiPr2 complexes can be considered as an intermediate between the base-stabilized silylene and silyl cases, and therefore they have been proposed as 2-pyridone-stabilized iridium silylene/silyl bonds.
中文翻译:

2-吡啶酮稳定的铱甲硅烷基/甲硅烷基络合物:结构和QTAIM分析
铱(III通式)配合物的[Ir(X)(κ 2 -NSi IPR2)2 ](NSI IPR2 =(4-甲基-吡啶-2-基氧基)二异丙; X =氯,3 ; CF 3 SO 3,5 ; CF 3 CO 2,6)已经制备和完全表征,包括X射线衍射研究和理论计算。硅原子上异丙基取代基的存在有利于配合物3和5中的单体结构。短的Ir-Si键距离(2.25-2.28埃)表示在一定程度上的Ir-Si键的碱稳定的亚甲硅基字符的3,5和6由2-吡啶酮部分协助。但是,这些Ir-Si键的缩短可能是2-吡啶酮几何结构受约束的结果,因此不能排除这些键的甲硅烷基特性。DFT理论研究了对于复杂3的Ir-Si键的性质以及其他四个铱配合物,找到了Ir和Si原子之间不同键合情况的代表实例:甲硅烷基,碱辅助的甲硅烷基(均具有阴离子碱和中性碱)和甲硅烷基键,并利用电子电荷密度。这些研究的结果表明,Ir–NSi iPr2配合物中的Ir–Si键可被认为是碱稳定的甲硅烷基和甲硅烷基之间的中间物,因此,它们被建议用作2-吡啶酮稳定的铱甲硅烷基/甲硅烷基。债券。
更新日期:2020-11-25
中文翻译:

2-吡啶酮稳定的铱甲硅烷基/甲硅烷基络合物:结构和QTAIM分析
铱(III通式)配合物的[Ir(X)(κ 2 -NSi IPR2)2 ](NSI IPR2 =(4-甲基-吡啶-2-基氧基)二异丙; X =氯,3 ; CF 3 SO 3,5 ; CF 3 CO 2,6)已经制备和完全表征,包括X射线衍射研究和理论计算。硅原子上异丙基取代基的存在有利于配合物3和5中的单体结构。短的Ir-Si键距离(2.25-2.28埃)表示在一定程度上的Ir-Si键的碱稳定的亚甲硅基字符的3,5和6由2-吡啶酮部分协助。但是,这些Ir-Si键的缩短可能是2-吡啶酮几何结构受约束的结果,因此不能排除这些键的甲硅烷基特性。DFT理论研究了对于复杂3的Ir-Si键的性质以及其他四个铱配合物,找到了Ir和Si原子之间不同键合情况的代表实例:甲硅烷基,碱辅助的甲硅烷基(均具有阴离子碱和中性碱)和甲硅烷基键,并利用电子电荷密度。这些研究的结果表明,Ir–NSi iPr2配合物中的Ir–Si键可被认为是碱稳定的甲硅烷基和甲硅烷基之间的中间物,因此,它们被建议用作2-吡啶酮稳定的铱甲硅烷基/甲硅烷基。债券。









































 京公网安备 11010802027423号
京公网安备 11010802027423号