当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational editing of intrinsically disordered protein by α-methylation
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-04 , DOI: 10.1039/d0sc04482b Valentin Bauer 1 , Boris Schmidtgall 1 , Gergő Gógl 2, 3 , Jozica Dolenc 4 , Judit Osz 2 , Yves Nominé 2, 3 , Camille Kostmann 2, 3 , Alexandra Cousido-Siah 2, 3 , André Mitschler 2, 3 , Natacha Rochel 2 , Gilles Travé 2, 3 , Bruno Kieffer 2 , Vladimir Torbeev 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-11-04 , DOI: 10.1039/d0sc04482b Valentin Bauer 1 , Boris Schmidtgall 1 , Gergő Gógl 2, 3 , Jozica Dolenc 4 , Judit Osz 2 , Yves Nominé 2, 3 , Camille Kostmann 2, 3 , Alexandra Cousido-Siah 2, 3 , André Mitschler 2, 3 , Natacha Rochel 2 , Gilles Travé 2, 3 , Bruno Kieffer 2 , Vladimir Torbeev 1
Affiliation
|
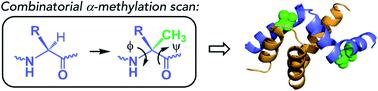
Intrinsically disordered proteins (IDPs) constitute a large portion of “Dark Proteome” – difficult to characterize or yet to be discovered protein structures. Here we used conformationally constrained α-methylated amino acids to bias the conformational ensemble in the free unstructured activation domain of transcriptional coactivator ACTR. Different sites and patterns of substitutions were enabled by chemical protein synthesis and led to distinct populations of α-helices. A specific substitution pattern resulted in a substantially higher binding affinity to nuclear coactivator binding domain (NCBD) of CREB-binding protein, a natural binding partner of ACTR. The first X-ray structure of the modified ACTR domain - NCBD complex visualized a unique conformation of ACTR and confirmed that the key α-methylated amino acids are localized within α-helices in the bound state. This study demonstrates a strategy for characterization of individual conformational states of IDPs.
中文翻译:

通过α-甲基化对内在无序蛋白进行构象编辑
内在无序的蛋白质(IDP)构成了“深色蛋白质组”的大部分-难以表征或尚未发现的蛋白质结构。在这里,我们使用构象约束的α-甲基化氨基酸来偏向转录共激活因子ACTR的自由非结构化激活域中的构象集合。化学蛋白质合成可实现不同的取代位点和取代模式,并导致不同的α螺旋种群。特定的取代模式导致与CREB结合蛋白(ACTR的天然结合伴侣)的核共激活因子结合域(NCBD)的结合亲和力大大提高。修饰的ACTR域-NCBD复合物的第一个X射线结构显示了ACTR的独特构象,并确认关键的α-甲基化氨基酸以结合状态位于α-螺旋内。这项研究展示了一种表征IDP个体构象状态的策略。
更新日期:2020-11-25
中文翻译:

通过α-甲基化对内在无序蛋白进行构象编辑
内在无序的蛋白质(IDP)构成了“深色蛋白质组”的大部分-难以表征或尚未发现的蛋白质结构。在这里,我们使用构象约束的α-甲基化氨基酸来偏向转录共激活因子ACTR的自由非结构化激活域中的构象集合。化学蛋白质合成可实现不同的取代位点和取代模式,并导致不同的α螺旋种群。特定的取代模式导致与CREB结合蛋白(ACTR的天然结合伴侣)的核共激活因子结合域(NCBD)的结合亲和力大大提高。修饰的ACTR域-NCBD复合物的第一个X射线结构显示了ACTR的独特构象,并确认关键的α-甲基化氨基酸以结合状态位于α-螺旋内。这项研究展示了一种表征IDP个体构象状态的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号