当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalysed chemoselective C-3 alkylation of indoles with alcohols through a borrowing hydrogen method
Chemical Communications ( IF 4.3 ) Pub Date : 2020-11-11 , DOI: 10.1039/d0cc07169b Amreen K. Bains 1, 2, 3, 4, 5 , Ayanangshu Biswas 1, 2, 3, 4, 5 , Debashis Adhikari 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2020-11-11 , DOI: 10.1039/d0cc07169b Amreen K. Bains 1, 2, 3, 4, 5 , Ayanangshu Biswas 1, 2, 3, 4, 5 , Debashis Adhikari 1, 2, 3, 4, 5
Affiliation
|
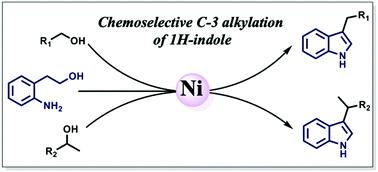
An inexpensive, air-stable, isolable nickel catalyst is reported that can perform chemoselective C3-alkylation of indoles with a variety of alcohols following “borrowing hydrogen”. A one-pot, cascade C3-alkylation starting from 2-aminophenyl ethyl alcohols, and thus obviating the need for pre-synthesized indoles, further adds to the broad scope of this method. The reaction is radical-mediated, and is significantly different from other examples, often dictated by metal–ligand bifunctionality.
中文翻译:

通过借位氢法用醇催化镍催化的吲哚与醇的化学选择C-3烷基化
据报道,一种廉价的,空气稳定的,可分离的镍催化剂可在“借入氢”后与多种醇进行吲哚的化学选择性C3-烷基化。从2-氨基苯基乙醇开始的一锅级联C3-烷基化反应,从而消除了对预合成的吲哚的需要,进一步扩大了该方法的范围。该反应是自由基介导的,与其他例子有显着差异,通常由金属-配体双官能度决定。
更新日期:2020-11-25
中文翻译:

通过借位氢法用醇催化镍催化的吲哚与醇的化学选择C-3烷基化
据报道,一种廉价的,空气稳定的,可分离的镍催化剂可在“借入氢”后与多种醇进行吲哚的化学选择性C3-烷基化。从2-氨基苯基乙醇开始的一锅级联C3-烷基化反应,从而消除了对预合成的吲哚的需要,进一步扩大了该方法的范围。该反应是自由基介导的,与其他例子有显着差异,通常由金属-配体双官能度决定。










































 京公网安备 11010802027423号
京公网安备 11010802027423号