当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conjecture on the Design of Helical Proteins
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.jpcb.0c05669 John J Kozak 1 , Harry B Gray 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.jpcb.0c05669 John J Kozak 1 , Harry B Gray 2
Affiliation
|
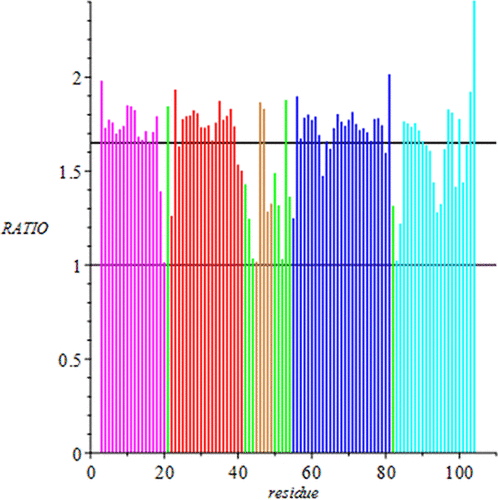
In an important advance in our understanding of protein folding, Wolynes and Onuchic found that the frustration ratio, Tf/Ts, for funneled energy landscapes is Tf/Ts ∼1.6. In our recent work on four heme proteins, we showed that when a protein unfolds from the native state to an early unfolded state, the degree of departure is characterized by a ratio f ∼1.6, where f is a measure of the elongation of n-residue segments of the polypeptide chain. Our analysis, which accounts for this apparent similarity in calculated signatures, is based on a logistic-map model of unfolding. We offer an important take home for the de novo protein synthesis community: in order to increase the probability of obtaining good quality crystals, nearest-neighbor repulsive interactions between adjacent residues (or sequences of residues) in the polypeptide chain must be propagated correctly.
中文翻译:

关于螺旋蛋白设计的猜想
Wolynes 和 Onuchic 发现,漏斗式能量景观的挫折比T f / T s是我们对蛋白质折叠理解的一个重要进展,T f / T s ∼1.6。在我们最近对四种血红素蛋白的研究中,我们表明,当蛋白质从天然状态展开到早期未折叠状态时,偏离程度的特征在于比率f ∼1.6,其中f是n伸长的量度-多肽链的残基片段。我们的分析基于展开的逻辑地图模型,解释了计算签名中的这种明显相似性。我们为从头蛋白质合成社区提供了一个重要的收获:为了增加获得优质晶体的可能性,多肽链中相邻残基(或残基序列)之间的最近邻排斥相互作用必须正确传播。
更新日期:2020-12-10
中文翻译:

关于螺旋蛋白设计的猜想
Wolynes 和 Onuchic 发现,漏斗式能量景观的挫折比T f / T s是我们对蛋白质折叠理解的一个重要进展,T f / T s ∼1.6。在我们最近对四种血红素蛋白的研究中,我们表明,当蛋白质从天然状态展开到早期未折叠状态时,偏离程度的特征在于比率f ∼1.6,其中f是n伸长的量度-多肽链的残基片段。我们的分析基于展开的逻辑地图模型,解释了计算签名中的这种明显相似性。我们为从头蛋白质合成社区提供了一个重要的收获:为了增加获得优质晶体的可能性,多肽链中相邻残基(或残基序列)之间的最近邻排斥相互作用必须正确传播。











































 京公网安备 11010802027423号
京公网安备 11010802027423号