当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature Dependence of Aqueous-Phase Decomposition of α-Hydroxyalkyl-Hydroperoxides
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.jpca.0c09862 Mingxi Hu 1 , Kunpeng Chen 2 , Junting Qiu 1 , Ying-Hsuan Lin 2 , Kenichi Tonokura 1 , Shinichi Enami 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.jpca.0c09862 Mingxi Hu 1 , Kunpeng Chen 2 , Junting Qiu 1 , Ying-Hsuan Lin 2 , Kenichi Tonokura 1 , Shinichi Enami 3
Affiliation
|
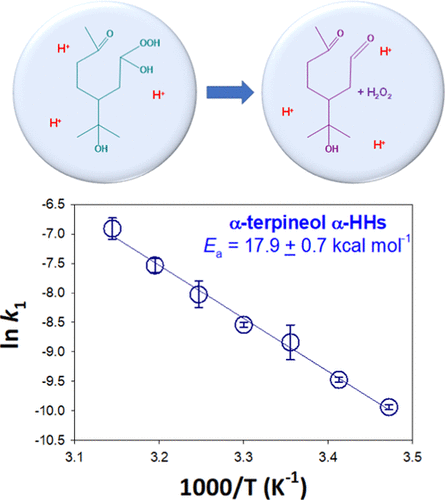
Ozonolysis of unsaturated organic species with water produces α-hydroxyalkyl-hydroperoxides (α-HHs), which are reactive intermediates that lead to the formation of H2O2 and multifunctionalized species in atmospheric condensed phases. Here, we report temperature-dependent rate coefficients (k) for the aqueous-phase decomposition of α-terpineol α-HHs at 283–318 K and terpinen-4-ol α-HHs at 313–328 K. The temporal profiles of α-HH signals, detected as chloride adducts by negative-ion electrospray mass spectrometry, showed single-exponential decay, and the derived first-order k for α-HH decomposition increased as temperature increased, e.g., k(288 K) = (4.7 ± 0.2) × 10–5, k(298 K) = (1.5 ± 0.4) × 10–4, k(308 K) = (3.4 ± 0.9) × 10–4, k(318 K) = (1.0 ± 0.2) × 10–3 s–1 for α-terpineol α-HHs at pH 6.1. Arrhenius plot analysis yielded activation energies of 17.9 ± 0.7 (pH 6.1) and 17.1 ± 0.2 kcal mol–1 (pH 6.2) for the decomposition of α-terpineol and terpinen-4-ol α-HHs, respectively. Activation energies of 18.6 ± 0.2 and 19.2 ± 0.5 kcal mol–1 were also obtained for the decomposition of α-terpineol α-HHs in acidified water at pH 5.3 and 4.5, respectively. Theoretical kinetic and thermodynamic calculations confirmed that both water-catalyzed and proton-catalyzed mechanisms play important roles in the decomposition of these α-HHs. The relatively strong temperature dependence of k suggests that the lifetime of these α-HHs in aqueous phases (e.g., aqueous aerosols, fog, cloud droplets, wet films) is controlled not only by the water content and pH but also by the temperature of these media.
中文翻译:

α-羟基烷基-羟基过氧化物的水相分解的温度依赖性
用水对不饱和有机物进行臭氧分解会生成α-羟烷基氢过氧化物(α-HHs),它们是反应性中间体,可导致在大气冷凝相中形成H 2 O 2和多官能团物质。在这里,我们报告了在283–318 K时α-松油醇α-HHs和在313–328 K时松油烯-4-醇α-HHs的水相分解的温度依赖性速率系数(k)。 -HH信号(通过负离子电喷雾质谱法检测为氯化物加合物)显示单指数衰减,并且随温度升高,推导的用于α-HH分解的一阶k增加,例如,k(288 K)=(4.7± 0.2)×10 –5,k(298K)=(1.5±0.4)×10 -4,ķ(308 K)=(3.4±0.9)×10 -4,ķ(318 K)=(1.0±0.2)×10 -3小号-1为pH值为6.1的α-松油醇α-HHs。Arrhenius图分析得出的α-萜品醇和萜品醇-4-醇α-HHs的分解活化能分别为17.9±0.7(pH 6.1)和17.1±0.2 kcal mol –1(pH 6.2)。活化能为18.6±0.2和19.2±0.5 kcal mol –1还获得了分别在pH 5.3和4.5的酸化水中分解α-萜品醇α-HHs的方法。理论动力学和热力学计算证实,水催化的和质子催化的机理在这些α-HHs的分解中均起重要作用。k的相对较强的温度依赖性表明,这些α-HH在水相(例如,气溶胶,雾,云滴,湿膜)中的寿命不仅受含水量和pH值的控制,而且还受这些温度的控制。媒体。
更新日期:2020-12-10
中文翻译:

α-羟基烷基-羟基过氧化物的水相分解的温度依赖性
用水对不饱和有机物进行臭氧分解会生成α-羟烷基氢过氧化物(α-HHs),它们是反应性中间体,可导致在大气冷凝相中形成H 2 O 2和多官能团物质。在这里,我们报告了在283–318 K时α-松油醇α-HHs和在313–328 K时松油烯-4-醇α-HHs的水相分解的温度依赖性速率系数(k)。 -HH信号(通过负离子电喷雾质谱法检测为氯化物加合物)显示单指数衰减,并且随温度升高,推导的用于α-HH分解的一阶k增加,例如,k(288 K)=(4.7± 0.2)×10 –5,k(298K)=(1.5±0.4)×10 -4,ķ(308 K)=(3.4±0.9)×10 -4,ķ(318 K)=(1.0±0.2)×10 -3小号-1为pH值为6.1的α-松油醇α-HHs。Arrhenius图分析得出的α-萜品醇和萜品醇-4-醇α-HHs的分解活化能分别为17.9±0.7(pH 6.1)和17.1±0.2 kcal mol –1(pH 6.2)。活化能为18.6±0.2和19.2±0.5 kcal mol –1还获得了分别在pH 5.3和4.5的酸化水中分解α-萜品醇α-HHs的方法。理论动力学和热力学计算证实,水催化的和质子催化的机理在这些α-HHs的分解中均起重要作用。k的相对较强的温度依赖性表明,这些α-HH在水相(例如,气溶胶,雾,云滴,湿膜)中的寿命不仅受含水量和pH值的控制,而且还受这些温度的控制。媒体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号