当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, Characterization, and Catalytic Activity of Bimetallic Ti/Cr Complexes
Organometallics ( IF 2.5 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.organomet.0c00645 Jiawei Chen 1 , Hunter B. Vibbert 1 , Chengbo Yao 1 , Amymarie K. Bartholomew 1 , Alexander P. Aydt 1 , Steffen Jockusch 1 , Jack R. Norton 1 , Matthew Hammond 1 , Michael Rauch 1
Organometallics ( IF 2.5 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.organomet.0c00645 Jiawei Chen 1 , Hunter B. Vibbert 1 , Chengbo Yao 1 , Amymarie K. Bartholomew 1 , Alexander P. Aydt 1 , Steffen Jockusch 1 , Jack R. Norton 1 , Matthew Hammond 1 , Michael Rauch 1
Affiliation
|
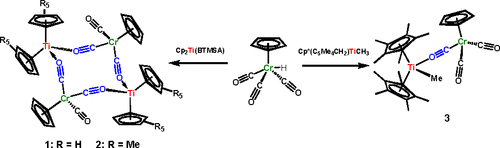
We report herein the reactions of the Rosenthal complexes, Cp2Ti(BTMSA) and Cp*2Ti(BTMSA) (BTMSA: bis(trimethylsilyl)acetylene), with CpCr(CO)3H. Those complexes are known to dissociate their alkyne ligands and to exhibit the reactivities of the putative titanocenes “Cp2Ti” and “Cp*2Ti”. Our reactions appear to proceed through the addition of CpCr(CO)3H to these titanocenes, giving rise to TiIV-H intermediates that lose H2 to form the TiIII–Cr complexes 1 and 2. In the solid state, 1 and 2 both adopt a dimeric geometry, involving a 12-membered “Ti2Cr2” ring held together with Ti–O–C–Cr bridging carbonyls. The terminal carbonyls in 1 and 2 are trans. DFT calculations confirm that the loss of H2 from the TiIV-H intermediate—forming the TiIII-Cr dimer—is exergonic by 14.2 kcal mol–1 in the gas phase. The Bercaw compound Cp* (C5Me4CH2)TiCH3 reacts with CpCr(CO)3H to form a TiIV-CH3 species with a coordinated CpCr(CO)3– anion (3). The Ti-Cr dimer 1 is capable of catalyzing the hydrogenation of an epoxide to an anti-Markovnikov alcohol, avoiding the use of Cp2TiX2 (X = Cl– or mesylate), NaCpCr(CO)3, and HCpCr(CO)3 separately as in our previous catalyst system.
中文翻译:

双金属Ti / Cr配合物的合成,表征和催化活性
我们在此报告Rosenthal配合物,Cp 2 Ti(BTMSA)和Cp * 2 Ti(BTMSA)(BTMSA:双(三甲基甲硅烷基)乙炔)与CpCr(CO)3 H的反应。已知这些配合物可解离其炔烃并表现出推定的钛烷“ Cp 2 Ti”和“ Cp * 2 Ti”的反应性。我们的反应似乎是通过向这些钛钛烯中添加CpCr(CO)3 H来进行的,从而生成失去H 2的Ti IV -H中间体,从而形成Ti III -Cr络合物1和2。在固态时1和2两者均采用二聚体几何结构,包括一个12元的“ Ti 2 Cr 2 ”环与Ti–OC–Cr桥连羰基结合在一起。1和2中的末端羰基是反式的。DFT计算证实,在气相中Ti IV -H中间体(形成Ti III -Cr二聚体)中的H 2损失为14.2 kcal mol –1。所述Bercaw化合物的Cp *(C 5我4 CH 2)TICH 3层发生反应以CPCR(CO)3 H至形成的Ti IV -CH 3种具有协调CPCR(CO)3–阴离子(3)。所述钛铬二聚体1是能够催化环氧化物与抗马氏醇的氢化,避免了使用Cp的的2 TIX 2(X =氯-或甲磺酸酯),NaCpCr(CO)3,和HCpCr(CO)与我们以前的催化剂系统一样,分别为3。
更新日期:2020-12-28
中文翻译:

双金属Ti / Cr配合物的合成,表征和催化活性
我们在此报告Rosenthal配合物,Cp 2 Ti(BTMSA)和Cp * 2 Ti(BTMSA)(BTMSA:双(三甲基甲硅烷基)乙炔)与CpCr(CO)3 H的反应。已知这些配合物可解离其炔烃并表现出推定的钛烷“ Cp 2 Ti”和“ Cp * 2 Ti”的反应性。我们的反应似乎是通过向这些钛钛烯中添加CpCr(CO)3 H来进行的,从而生成失去H 2的Ti IV -H中间体,从而形成Ti III -Cr络合物1和2。在固态时1和2两者均采用二聚体几何结构,包括一个12元的“ Ti 2 Cr 2 ”环与Ti–OC–Cr桥连羰基结合在一起。1和2中的末端羰基是反式的。DFT计算证实,在气相中Ti IV -H中间体(形成Ti III -Cr二聚体)中的H 2损失为14.2 kcal mol –1。所述Bercaw化合物的Cp *(C 5我4 CH 2)TICH 3层发生反应以CPCR(CO)3 H至形成的Ti IV -CH 3种具有协调CPCR(CO)3–阴离子(3)。所述钛铬二聚体1是能够催化环氧化物与抗马氏醇的氢化,避免了使用Cp的的2 TIX 2(X =氯-或甲磺酸酯),NaCpCr(CO)3,和HCpCr(CO)与我们以前的催化剂系统一样,分别为3。









































 京公网安备 11010802027423号
京公网安备 11010802027423号